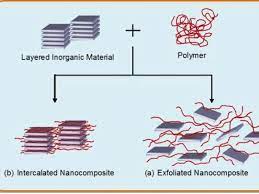
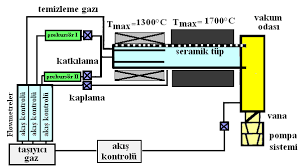
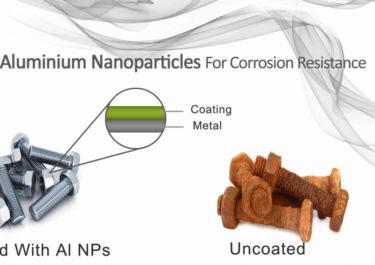
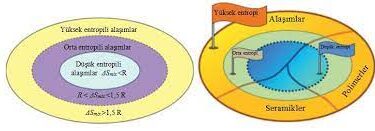
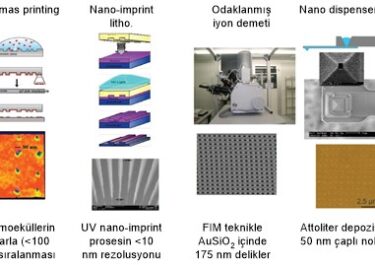
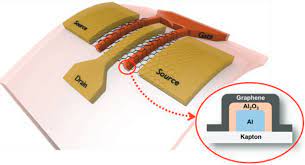
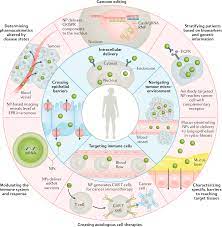
Carbides Atomic Structure to Industrial Applications
**Atomic Structure:** Carbides are compounds formed by the combination of carbon with another element, typically a metal or metalloid. The atomic structure of carbides can vary depending on the type of carbide and the bonding between carbon atoms and the other element. Here are some common examples: 1. **Binary Carbides:** These carbides consist of carbon