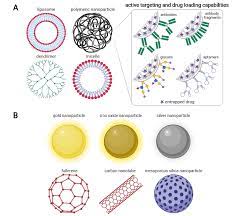
Organic and inorganic nanoparticles are materials of two or more dimensions, with a size in the range of 1–100
nm. Nanoparticles show unique size dependent physical and chemical properties, for example, optical,
magnetic, catalytic, thermodynamic and electrochemical. The chemical composition and the shape of a
nanoparticle also influence its specific properties. Nanoparticles are prepared with organic polymers (organic
nanoparticles) and/or inorganic elements (inorganic nanoparticles). Liposomes, dendrimers, carbon
nanomaterials and polymeric micelles are examples of organic nanoparticles.
Organic and inorganic nanoparticles: Liposomes are phospholipid vesicles (50–100 nm) that have a bilayer
membrane structure similar to that of biological membranes and an internal aqueous phase. Liposomes are
classified according to size and number of layers into multi-, oligo- or uni-lamellar. Their amphiphilic nature
enables liposomes to transport hydrophilic drugs entrapped within their aqueous interior and hydrophobic
drugs dissolved into the membrane. Owing to their physicochemical characteristics, liposomes show excellent
circulation, penetration and diffusion properties. Moreover, the liposome surface can be modified with ligands
and/or polymers to increase drug delivery specificity
Organic and inorganic nanoparticles
Dendrimers are highly branched synthetic polymers (<15 nm) with layered architectures constituted of a central
core, an internal region and numerous terminal groups that determine dendrimer characteristics. A dendrimer
can be prepared using multiple types of chemistry, the nature of which defines the dendrimer solubility and
biological activity. Dendrimers show intrinsic drug properties and are used as tissue-repair scaffolds.
Moreover,dendrimers are excellent drug and imaging diagnosis-agent carriers through chemical modification of
their multiple terminal groups
organic and inorganic nanoparticles
Carbon nanotubes Carbon nanotubes belong to the family of fullerenes and are formed of coaxial graphite
sheets (<100 nm) rolled up into cylinders. Thesestructures can be obtained either as single- (one graphite sheet)
or multi-walled nanotubes (several concentric graphite sheets). They exhibit excellent strength and electrical
properties and are efficient heat conductors. Owing to their metallic or semiconductor nature, nanotubes are
often used as biosensors. Carbon nanotubes can be rendered water soluble by surface functionalisation.
Therefore, they are also used as drug carriers and tissue-repair scaffolds. Inorganic nanoparticles, such as quantum dots, polystyrene, magnetic, ceramic and metallic nanoparticles, have a central core composed of inorganic materials that define their fluorescent, magnetic, electronic and optical properties.
Organic and inorganic nanoparticles
Quantum dots Quantum dots are colloidal fluorescent semiconductor nanocrystals (2–10 nm). The central core
of quantum dots consists of combinations of elements from groups II–VI of the periodic system (CdSe, CdTe,
CdS, PbSe, ZnS and ZnSe) or III–V (GaAs, GaN, InP and InAs), which are ‘overcoated’ with a layer of ZnS.
Quantum dots are photostable. They show size- and composition-tuneable emission spectra and high quantum
yield. They are resistant to photobleaching and show exceptional resistance to photo and chemical degradation.
All these characteristics make quantum dots excellent contrast agents for imaging and labels for bioassays.
