Inert Gas Condensation for Nanoparticle Synthesis
Nanoparticles have garnered significant attention in recent years due to their unique properties and a wide range of applications in fields such as electronics, medicine, energy storage, and environmental science. The synthesis of these nanoparticles often requires specific techniques that allow for precise control over their size, shape, and composition. One such technique is inert gas condensation (IGC), a versatile and widely used method for synthesizing metal and metal oxide nanoparticles, as well as composite materials at the nanoscale.
In this article, we will explore the principles, process, advantages, and applications of inert gas condensation as a method for nanoparticle synthesis.
What is Inert Gas Condensation (IGC)?
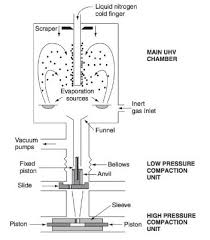
Inert Gas Condensation (IGC) is a physical vapor deposition (PVD) technique that involves the production of nanoparticles by condensing vaporized material in the presence of an inert gas, typically argon or helium, under controlled conditions. This method allows for the precise generation of nanoparticles in a vacuum environment without the need for chemical reactions.
The IGC process takes place in a vacuum chamber, where a solid material (such as metal or metal alloy) is evaporated or sublimated by heating. The evaporated atoms or molecules then cool down in the presence of an inert gas, leading to the formation of nanoparticles. These nanoparticles can be collected on a substrate or within the chamber.
Key Features of IGC:
- Use of an inert gas: The inert gas prevents chemical reactions during the condensation process, ensuring that the nanoparticles remain pure and free from contamination.
- Control over size and distribution: By adjusting parameters like gas pressure, evaporation rate, and cooling rate, the size and morphology of the nanoparticles can be fine-tuned.
- Production of high-purity nanoparticles: Since the process is physical and does not involve chemicals, the produced nanoparticles are generally of high purity.
The Process of Inert Gas Condensation
The IGC process involves several critical steps that result in the formation of nanoparticles:
1. Vaporization of Material
- A solid material (typically a metal, alloy, or ceramic) is heated in a vacuum chamber to a temperature where it evaporates or sublimates. The material is usually heated by resistive heating or a laser to vaporize the substance.
2. Nucleation
- Once the material has vaporized, it is carried by the flow of an inert gas (like argon, helium, or neon) into a cooler region of the chamber. The rapid cooling causes the vaporized material to condense, forming nucleated clusters or small droplets of material.
3. Growth of Nanoparticles
- The nucleated clusters grow by a process of collisional aggregation or atom-by-atom deposition, depending on the conditions in the chamber. By controlling the pressure of the inert gas and the temperature of the chamber, the size and uniformity of the nanoparticles can be controlled.
4. Collection and Post-Treatment
- The nanoparticles are collected on a substrate or a collection surface. The size distribution and morphology of the particles can be influenced by various parameters, such as the gas flow rate, pressure, and cooling rate. In some cases, post-treatment processes such as annealing or surface functionalization are used to further modify the properties of the nanoparticles.
Advantages of Inert Gas Condensation
IGC offers several unique advantages, which make it an attractive method for nanoparticle synthesis:
1. High Purity of Nanoparticles
- Since the process does not involve chemical precursors or solvents, the produced nanoparticles are typically free from impurities and contaminants. This makes IGC an excellent choice for applications requiring high-purity nanoparticles, such as electronics and biomedical devices.
2. Control Over Particle Size and Morphology
- One of the key benefits of IGC is the ability to precisely control the size, shape, and distribution of nanoparticles. By adjusting the gas pressure, temperature, and deposition rate, the particle size can be tuned from just a few nanometers to hundreds of nanometers, enabling the creation of monodisperse nanoparticle populations.
3. Versatility in Material Selection
- IGC is a highly versatile technique and can be used to produce nanoparticles from a variety of materials, including metals, metal oxides, alloys, and semiconductors. The technique is flexible enough to handle a wide range of materials with varying volatility, enabling its use for many different applications.
4. Scalable for Industrial Production
- IGC can be scaled for industrial production of nanoparticles. While laboratory setups are typically small and capable of producing nanoparticle samples, larger vacuum chambers and improved deposition methods allow for continuous production of nanoparticles in bulk, which is essential for commercial applications.
5. No Need for Chemical Precursors
- IGC is a chemical-free method, meaning no chemical reagents or solvents are involved in the process. This eliminates the risk of unwanted chemical reactions and ensures that the nanoparticles are free from chemical contaminants, which is particularly important for applications in medicine, pharmaceuticals, and electronics.
Applications of Inert Gas Condensation for Nanoparticle Synthesis
The nanoparticles produced by inert gas condensation find applications across a wide array of industries. Some of the most notable applications include:
1. Nanomaterials for Electronics
- IGC is widely used to synthesize metal nanoparticles, such as gold, silver, platinum, and copper. These nanoparticles are used in nanoelectronics, sensors, and catalysts. In particular, nanoparticles made of gold and silver are highly valued for their optical properties and electrical conductivity, making them useful in photodetectors, surface-enhanced Raman spectroscopy (SERS), and photovoltaic devices.
2. Catalysis
- Nanoparticles, especially metal nanoparticles (like platinum, palladium, and rhodium), exhibit unique catalytic properties due to their high surface area and reactivity. IGC is often used to synthesize these nanoparticles for use in various catalytic processes, such as hydrogenation, oxidation, and fuel cells.
3. Energy Storage
- Nanoparticles are increasingly used in energy storage devices like lithium-ion batteries and supercapacitors. The high surface area and conductivity of metal nanoparticles synthesized through IGC make them ideal for enhancing the performance of electrode materials in energy storage devices.
4. Biomedical Applications
- In biomedicine, metal oxide nanoparticles produced via IGC are being explored for use in drug delivery systems, biomarkers, and imaging agents. Additionally, silver nanoparticles, with their antimicrobial properties, are used in wound dressings, medical implants, and antibacterial coatings.
5. Environmental Remediation
- Metal nanoparticles, such as iron and copper nanoparticles, produced by IGC, are being employed in environmental cleanup applications, such as the remediation of contaminated water and soil decontamination. These nanoparticles are effective at degrading organic pollutants and heavy metals.
6. Nanocomposites
- The synthesis of nanocomposites, which are materials that combine nanoparticles with other materials (e.g., polymers, ceramics, and metals), is a rapidly growing area. IGC is used to produce nanoparticles that can be incorporated into lightweight and high-strength nanocomposites for aerospace, automotive, and construction applications.
Challenges of Inert Gas Condensation
While inert gas condensation has many advantages, there are some challenges associated with the process:
1. High Equipment Costs
- The need for a vacuum chamber, precise temperature control, and a supply of inert gases can make IGC equipment relatively expensive. This can be a barrier for smaller research labs or companies looking to adopt this technology for nanoparticle synthesis.
2. Limited Control Over Nanoparticle Shape
- While IGC allows for good control over the size and distribution of nanoparticles, controlling the shape of nanoparticles (such as creating nanowires or nanorods) can still be difficult without additional post-synthesis treatment or template-based methods.
3. Production of Agglomerates
- During the condensation process, there is a risk that the nanoparticles may agglomerate or cluster together, which can affect the material’s properties. Controlling the cooling rate and pressure is crucial to minimizing this issue.
Conclusion
Inert gas condensation (IGC) is a powerful and widely used technique for the synthesis of high-quality nanoparticles. The ability to control nanoparticle size, morphology, and purity makes it an ideal method for producing materials used in applications such as electronics, catalysis, biomedicine, and energy storage.
While the technique offers numerous advantages, such as high-purity nanoparticles, scalability, and versatility in materials, challenges such as equipment costs and control over nanoparticle shape remain. As technology advances and new materials are explored, the potential applications of IGC in nanomaterial synthesis are expected to continue to grow, contributing to innovation across multiple industries.
