Graphene blocks, which are two-dimensional, transverse sized are called graphene quantum dots (GQD), exhibiting physical, chemical, and biological properties.
GQD has a better prospect in the biomedical environment due to its small size (less than 100nm). Different studies showed that GQDs are less biotoxic and more biocompatible. Different methods are used for the synthesis of GQDs, further classified into two processes, top-down and bottom-up.
Introduction
The synthesis methods require a bulk of raw materials for processing, under high temperatures and pressure, which dope many elements or groups to synthesize GQDs. As compared to GQDs, carbon dots are spherical or semispherical particles of carbon less than 10nm in size, and are amorphous, whereas GQDs have crystalline. Much research has been conducted to identify GQDs properties and their application in different fields like biomedical, bioimaging, environmental protection, photothermal therapy, etc.
An Overview of Graphene Quantum Dots
Two-dimensional (2D) transverse size (less than 100nm) graphene blocks are called Graphene quantum dots (GQDs) and they have exceptional physical, chemical, and biological properties. A single atomic layer of carbon atoms is present in an ideal GQD. However, many synthesized GQDs also have functional groups like hydrogen and oxygen, and also consist of several atomic layers(less than 10nm in size).
The Better Prospect of Graphene Quantum Dots (GQDs)
In comparison to graphene or graphene oxide (GO), GQD has a better prospect in the biomedical field due to its small size. However, the main concerns regarding GQD before its practical application are their toxicity and biocompatibility. Different studies on GQDs have shown that they are biocompatible and have less bio toxicity. In a study by Xie et al., by using the lung cancer A549 cells as models, they studied the autophagy induction and cytotoxicity of three types of GQDs, which involve aGQDs (H2N-GQDs), cGQDs (HOOC-GQDs), hGQDs (HO-GQDs).
Results
The results have shown that the most toxic type of GQDs is hGQDs as it leads to significant cell death in hGQD concentration of 100 μg/mL, whereas the other types like aGQDs and cGQDs have shown no cytotoxicity within the range of measured concentration. Analysis of autophagy pathways has shown that all the GQDs can activate p-p38MAPK significantly, whereas aGQDs and hGQDs inhibit p-ERK1/2 but they can be activated by cGQDs. The p-JNK can be activated by hGQDs but can be inhibited by cGQDs and aGQDs. Whereas, hGQDs can activate Akt but it can be inhibited by aGQDs. The cytotoxicity of GQDs increases by the inhibiting impact of 3-MA on autophagy, it exhibits that there is a protective impact of autophagy on the GQDs toxicity.
Synthesis Methods of Graphene Quantum Dots (GQDs)
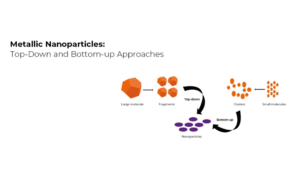
The current methods which are used for the synthesis of GQDs can be classified into two processes that are top-down and bottom-up. For the bottom-up processes, there’s a requirement for specific organic material and complex reaction steps, which makes exhibits difficulty in the optimization of conditions. Therefore, top-down processes are preferable to bottom-up processes in which large blocks of carbon materials are cut down into small pieces. A bulk of carbon materials is used as raw material in this method, which is easily available and more affordable and this method is more reliable for the synthesis of GQDs.
Top-Down Strategy
Based on top-down processes, there are several methods for the synthesis of GQDs, which include the chemical oxidation method, ultrasonic-assisted method, chemical vapor deposition method (CVD), hydrothermal method, electrochemical oxidation method, and pulsed laser ablation technique (PLA) or a combination of above-mentioned approaches.
Chemical Oxidation Method
The chemical oxidation method also referred to as the oxidation cutting method, is a vastly used method, in which graphene’s carbon bonds, Graphene Oxide, or carbon nanotubes are mostly destroyed by Sulphuric acid (H2SO4), Nitric acid (HNO3), or other oxidants.
Experimental System
An experimental system was developed by Liu et al., in which Vulcan XC-72 carbon black was used as a carbon source, and concentrated nitric acid reflux, which is a strong oxidant was used to synthesize GQDs of high purity. The purity and yield of GQDs were 99.96 wt % and 75 wt % respectively. The prepared GQDs showed multicolor photoluminescence (PL) from green to red on different wavelengths of excitation.
Introduction of Metal Impurities
Lu et al. prepared GQDs with hydrogen peroxide as the oxidant and black carbon as a precursor by using the hydrothermal method, this process was developed to introduce metal impurities and to avoid concentrated acids use. Synthesized GQDs have a diameter of 3.0 to 4.5 nm. It takes approximately 90 minutes for the whole synthesis process and it has several qualities including salt tolerance, good biocompatible, less toxicity, and also has good stability in the light. As compared to other reported methods, this method is considered fast and more green for the synthesis of GQDs. After this, Halder et al. made the use of GO as the precursor, oxidized and cracked it within 2 hours using hydrogen peroxide, to receive GQDs product and it also does not need any further purification method.
Use of Strong Oxidants
The chemical waste generated during the chemical oxidation method is not safe and is more likely to pollute the environment due to the use of some strong oxidants such as Sulphuric acid and nitric acid.
Hydrothermal Method
The most rapid and simple method for the synthesis of GQDs is the hydrothermal method. GQDs can be easily synthesized by using a variety of small molecular or macromolecular substances and these substances can be used as the starting material with high pressure and temperature. The principle of this process is to form GQDs by breaking the bonds between carbon substances and this can work with high pressure and temperature.
Use of Hydrogen Peroxide for The Synthesis of Graphene Quantum Dots
Tian et al. used a one-step solvothermal method to synthesize GDQs in N, N-dimethylformamide (DMF) area by using hydrogen peroxide (H2O2). In the whole process of preparation, concentrated nitric acid and sulphuric acid were not used for raw material, and impurities were also not introduced. By evaporation and filtration without dialysis, GQDs of high purity can be obtained. In the results of this process, the thickness and diameter of GQDs were within the range of 1-1.5nm and 20-40nm, respectively. 15% was the quantum yield (QY) under neutral circumstances. The PL signals showed better stability with different pH conditions, which clearly shows that it has vast application prospects in different conditions.
Advantages of The Method
There are many advantages of this method, which include high quantum yield, low cost, simple experimental setup, no requirement for dialysis, etc. The prepared GQDs showed better water solubility and were environment friendly, which represented their excellent applications in bioelectronics and the field of biomedical.
you can read our blog post here.
The Recent Synthesis of Graphene Quantum Dots
Zhang et al. have successfully prepared reduced graphene oxide quantum dots (rGOQDs) within 5 hours. The starting material to prepare graphene oxide was graphite, by using an improved Hummer’s method, and then for further hydrothermal treatment in a poly-lined autoclave at the temperature of 200 °C, DMF and GO were used as raw materials. 24.62% Quantum yield of synthesized GQDs was obtained, and nitrogen was extracted from DMF as of surface doping. Zebrafish were also studied by rGOQDs, which suggested valuable references for the biocompatible nature of bio-probes in vivo.
Full Use of Crop Biomass
For the full use of crop biomass, researchers used the hydrothermal method to produce high-quality GQDs, and for that they used rice husk as raw material. 15 wt% is the mass fraction of QY. The synthesized GQDs displayed sound colloidal water stability with adjustable and bright PL signals. Experiments showed that the synthesized GQDs are biocompatible and can be used for cell imaging as they can be easily translocated in the cytoplasm. In addition to this, during the synthesis of GQDs, mesoporous silica nanoparticles (MSNs) were synthesized as by-products.
Doping of Graphene Quantum Dots
To dope many elements or groups, the hydrothermal method can be used and a wide range of composites are used as raw material. moreover, another method to prepare different GQDs is by combining the hydrothermal method and chemical oxidation method. however, it takes a long time, usually 5 hours, and requires high pressure and temperature, which imposes safety issues.
Ultrasound-Assisted Method
A common method for the synthesis of material is by using ultrasonic technology. Numerous small bubbles will be formed in solutions by the action of ultrasound and the carbon-carbon bonds can be destroyed by the generated mechanical force.
Three Kinds of Graphene Quantum Dots
Gao et al. synthesized three types of GQDs expanded graphene quantum dots (EGQDs) pristine graphene quantum dots (PGQDs), and graphene oxide quantum dots (GOQDs), by using expanded graphite, natural graphite, and oxide graphite as the raw materials in a supercritical CO2/H2O system with the help of ultrasound. The results show that it is an environmentally friendly, low-cost, fast, and large-scale GDQs synthesis method, It can be an alternative way for the synthesis of various GQDs, especially PGQDs.
Electrochemical Oxidation Method
In the process of the electrochemical oxidation method, carbon-carbon bonds of graphene, graphite, or carbon nanotubes are oxidized and decomposed into various GQDs by using a high redox voltage (+ 1.5 to + 3 V).
Preparation of Crystalline Graphene Quantum Dots
Researchers have developed an electrochemical method with weak electrolytes (such as ammonia solution) for the oxidation and cutting process which results in the production of highly crystalline GQDs in aqueous systems in an efficient and controlled manner. The af-GQDs were synthesized by using a circular graphene paper as an anode and a Pt sheet as a cathode, for electrolyte, an ammonia solution (nitrogen source) was used, a voltage of 30V for at least 2 hours in an electrochemical cell. GQDs had 3 to 8 nm size and 28% of QY, which was 28 times stronger than that strong electrolyte (borax solution).
Meanwhile, GQDs show more crystallinity than bottom-up GQDs. By controlling the electrolyte concentration, the function of GQDs amino can also be controlled. In addition, these methods can be used with weak electrolytes and anode precursors to synthesize different kinds of GQDs.
Carbon Quantum Dot vs. Graphene Quantum Dot
The graphene quantum dots are also known as the GQDs where they work as disks of graphene and their size ranges from 2-20nms. As they have quantum confinement, a few defects in their surfaces and zigzag edges make them fluorescent. Their main composition is Sp2 hybridization of carbon as they are present in crystalline form. While on the other hand, carbon dots which are generally known as C-dots consist of spherical particles of carbon and their size range is always less than 10 nm. As explained earlier, their main composition is as of sp3 hybridization and amorphous in nature so they are capable of showing XRD of the basal planar of graphite.
Difference Between Carbon Quantum Dots and Graphene Quantum Dots
A lot of research is not specified about the differences in the GQDs with carbon quantum dots as most of them consider GQDs as one of the types of C-dots. This is the main reason why they are considered a family of quantum dots that are made out of carbon. At this stage too, their confinement is not understood well and is completely different as compared to the semiconductor quantum dots as in Cds or CdSe. This is due to the variation in their size and the tendency to have the same bandgap energy which is equivalent to 3.4 eV whereas in the case of pristine graphene it is either 0 eV or close to that only which makes it evident for them to be named as quantum dots when brought in comparison to graphene.
The graphene quantum dots and carbon dots are both zero in dimensional which is equivalent to 0-D. However, their hybridization is completely different as the hybridization for GQDs is in sp2 whereas for CDs it is in sp3. Also, GQDs are present in crystalline form and CDs are present in amorphous forms.
you can read our blog post here.
Applications of Graphene Quantum Dots (GQDs) in Drug Delivery
In recent years, applications for GQDs in the case of drug delivery, sensors, bio-imaging, hyperthermia, antibacterial, catalyst, and a lot of other areas have achieved worthy accomplishments. For applying GQDs to drug delivery, researchers have been conducted and they have opted for density functional theory calculations, molecular dynamics simulations, or a few other methods that could study the properties of GQDs.
Results
The results showed that AlN and AlP-doped GQDs could serve as potential carriers for FU drugs in the nanomedicine domain. Later, they [167] used DFT calculations to study the applications of GQDs and doped GQDs as potential carriers of isoniazid (Iso). The results confirmed that the AlN- and AlP-doped GQDs could be used as potential carriers for drug delivery applications. Recently, they [169] also studied the effects of different N-functionalities groups in the drug delivery performance of N-GQDs via DFT calculations and MD simulations. The drug release performance of the center N-GQDs is considered to be superior to that of pristine GQDs and edge N-GQDs. This review focuses on the research accomplishment of GQDs in drug delivery.
Different Ways of Drug Delivery
Drug delivery has a lot of ways but in the process, if we ignore the content of drug release then it is impossible to bring improvement to the therapeutic effect that the drugs are entitled to bring. That is why a lot of research is being carried out in the same regard so the relationship between drug delivery and drug release can be worked upon and the effects of drugs can be enhanced to a great level. In this way, their working efficiency is enhanced at a certain level which brings ease to all in the said area of work.
Use of Hydrothermal Method
A report was presented by Khodadadei et al. a few years back about the blue fluorescent nitrogen-doped graphene quantum dots also commonly known as N-GQDs as their synthesis is carried out by a hydrothermal method in which CA is used as a carbon source and urea is used as a nitrogen source. In this case, N-GQDs are said to be loaded with methotrexate (MTX) via a series of interactions that take place during the process. This study is a way of confirming the progress of GQDs for the drug-loaded cells as they work as the nanocarriers to prolong their cytotoxicity so that they are capable of killing cancer cells and provide the desired and maximum effect that these drugs are designed for.
Anti-Tumor Treatment
To attain the correct anti-tumor treatment, the first series of drugs are loaded on DDRs through the said interactions, and afterward, the tumors are to be targeted by the DDRs-loaded drugs via the interactions which are known as ligand-receptor interactions. The final step includes the release of these antitumor drugs in the lowest pH environment of tumor cells so that an effective tumor ablation can occur and adapt to the desired changes.
EPR-Photothermal Delivery-Release Mode
In the case of EPR-photo-thermal delivery release mode, DDRS works as either two-dimensional or three-dimensional but it is observed that it has no targeting function as it lacks a ligand. This entire movement is not controlled by a magnetic field as it also lacks magnetic iron oxide. When studying it generally, it is observed that it can be transferred to the tumor site via the EPR effect but once DDRS is released, it is said to be controlled via NIR radiation without completely relying on the present acidic environment.
Reduction in Drug Leakage
Several types of research have been conducted in the case of reducing drug leakage and bringing improvement to drug release efficiency in terms of tumor lesions. One of the researchers suggested that a DDRS can efficiently work for both purposes to be fulfilled. The study discovered that the molecularly imprinted polymers have a great effect on the loading efficiency of DOX and hence they are a great deal of help in reducing drug leakage. However, this step is considered one of the most essential steps in carrying out successful drug delivery as once the leakage has been minimized then the quality and quantity can be maximized more effectively and the necessary steps can be taken accordingly for improving the effective ways to enhance drug delivery.
Cell Imaging and Drug Delivery
UCNP known as the up-conversion nanoparticle works as the core and the GOQDs work as the shell. A research that was conducted, presented the synthesis of a core-shell nanoparticle for carrying out cell imaging and drug delivery. The first step of synthesis is carried out under the hydrothermal method in which the modification for the surface of UCNP is carried out to be transformed into polyethylene glycol 2 aminoethyl ether acetic acid. This leads to the synthesis of chemotherapeutic drugs and photosensitizers through a series of different interactions.
Conclusion
Graphene Quantum Dots are graphene blocks, two-dimensional, transverse in size(less than 100nm), and crystalline. GQDs exhibit exceptional properties and have better prospects in different environments and fields as compared to other substances like graphene or graphene oxide. The synthesis of GQDs takes place through different processes and methods. Hydrothermal has been considered the most rapid and simple method for GQDs synthesis but under many conditions, due to which it shows vast perspective in different fields such bioelectronics and biomedical. The better perspective and approach of GQDs are due to their properties.
