Gold Nanoparticles are emerging as promising agents for cancer therapy and are being investigated as drug
carriers, photothermal agents, contrast agents and radiosensitisers. This review introduces the field of
nanotechnology with a focus on recent gold Nanoparticles research which has led to early-phase clinical trials.
In particular, the pre-clinical evidence for gold Nanoparticles as sensitisers with ionising radiation in vitro and in
vivo at kilovoltage and megavoltage energies is discussed
Gold Nanoparticles Cancer Treatment Blog
For this therapy to be effective, the gold Nanoparticles have to attach to a cancer cell more easily than a healthy
cell, otherwise the laser pulse would damage healthy tissue. To accomplish this, the researchers coated the
particles in an antibody that is known to attach to the specific type of aggressive cancer they were using in the
study, head and neck squamous cell carcinoma. It is this antibody that attaches to the receptor at the cell
membrane.
Preparing the Nanoparticles
The researchers also found that there was an optimal size to the gold particles. If the particles were less then 10
billionths of a meter (10 nanometers) in diameter, the cell would quickly clear them out. If the particles were
greater than 100 nanometers the cell had trouble internalizing the particles. The scientists found that the
Nanoparticles which worked best for their study were around 60 billionths of a meter in diameter.
Gold Nanoparticles Cancer Treatment Blog
These antibody-coated gold Nano spheres were found to insert themselves into cancer cells far more readily
than healthy cells; the average cluster size in healthy cells was found to be 64 nanometers (about 1 sphere),
while the average cluster size in cancer cells was found to be about 300 nanometers (about 100 spheres).
One natural advantage to this process is that tumors often have leaky vascular systems, so when the gold
particles are injected intravenously near the known cancer, they rapidly spread and are incorporated throughout
the cancerous region. The scientists noted that 24 hours of time was needed after the injections to allow gold
clusters to form in the cel
Blowing Up the Cancer Cell
Once the Gold Nanoparticles are incorporated into cells, the researchers exposed the tissue to a laser pulse
(near infrared radiation of wavelength 782 nanometers) for a duration of 30 trillionths of a second (30
picoseconds). This particular type of laser light is optimal because it penetrates tissue well and it is not resonant
with the gold Nanoparticles. This means that when the light strikes the Nanoparticles it does not absorb it and
immediately start warming the bulk of the gold Nanoparticles resulting in overheating the cell. Rather, during
the first 10 nanoseconds some melting of the surface without bulk heating of the gold nanoparticles2 occurs,
and this vaporizes the fluid around the gold Nanoparticles. The vaporized fluid rapidly expands and then
collapses. However, it is imperative to note that the effect is inconsequential unless there are tens of
Nanoparticles in the cluster. The creation of a Nano bubble that rapidly expands and collapses, with enough
energy to destroy a cell, is dependent on the number of gold spheres in the cluster, with the severity increasing
as the cluster size increases
Gold Nanoparticles Cancer Treatment Blog
This selectivity of severity with size is what keeps the healthy cells safe. The gold Nanoparticles don’t do well at
transforming the laser pulse to thermal energy on their own, so any Nano bubbles formed are relatively
insignificant—a few spheres together in a healthy cell will not cause any damage. It is the cluster of
nanoparticles within the cancer cells that effectively converts the laser pulse to thermal energy, causing
vaporization of the surrounding fluid, a rapid expansion, and a collapse, leading to the destruction of the cancer
cell. This event is not easily detected by optical means, but it is easily “heard” by detecting the sound wave
produced during the rapid expansion and collapse.
Nanoparticles are currently employed in several medical applications and many more have been suggested, with
great potential benefits for patients and medical providers. Due to their high atomic mass, gold Nanoparticles
can absorb significantly more radiation than soft tissue cells, making them ideal for boosting the radiation dose
in tumors or enhancing contrast of specific tissues during diagnostic imaging (e.g. doping a tissue with 1% of its
weight with Nanoparticles would double the radiation dose absorbed following kV X-ray exposure).
Gold Nanoparticles Cancer Treatment Blog
It has been almost 4 decades since the “war on cancer” was declared. It is now generally believed that
personalized medicine is the future for cancer patient management. Possessing unprecedented potential for
early detection, accurate diagnosis, and personalized treatment of cancer, nanoparticles have been extensively
studied over the last decade. In this review, we will summarize the current state-of-the-art of gold nanoparticles
in biomedical applications targeting cancer. Gold nanospheres, nanorods, nanoshells, nanocages, and surface
enhanced Raman scattering nanoparticles will be discussed in detail regarding their uses in in vitro assays, ex
vivo and in vivo imaging, cancer therapy, and drug delivery. Multifunctionality is the key feature of nanoparticlebased agents. Targeting ligands, imaging labels, therapeutic drugs, and other functionalities can all be integrated to allow for targeted molecular imaging and molecular therapy of cancer. Big strides have been made
and many proof-of-principle studies have been successfully performed. The future looks brighter than ever yet
many hurdles remain to be conquered. A multifunctional platform based on gold nanoparticles, with multiple
receptor targeting, multimodality imaging, and multiple therapeutic entities, holds the promise for a “magic
gold bullet” against cancer
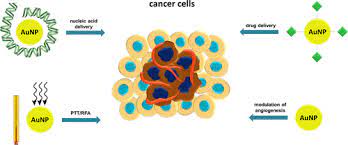
Biomedical applications of gold nanoparticles: Cancer nanotechnology is an interdisciplinary area with broad
potential applications in fi ghting cancer, including molecular imaging, molecular diagnosis, targeted therapy and bioinformatics. The continued development of cancer nanotechnology holds the promise for personalized oncology in which genetic and protein biomarkers can be used to diagnose and treat cancer based on the
molecular profi le of each individual patient. Gold nanoparticles have been investigated in diverse areas such as
in vitro assays, in vitro and in vivo imaging, cancer therapy, and drug delivery
Multifunctionality is the key advantage of nanoparticles over traditional approaches. Targeting ligands, imaging
labels, therapeutic drugs, and many other functional moieties can all be integrated into the nanoparticle
conjugate to allow for targeted molecular imaging and molecular therapy of cancer. Gold nanoparticle is unique
in a sense because of itsintriguing optical properties which can be exploited for both imaging and therapeutic
applications. The future of nanomedicine lies in multifunctional nanoplatforms which combine both therapeutic
components and multimodality imaging. The ultimate goal is that nanoparticle-based agents can allow for
efficient, specific in vivo delivery of drugs without systemic toxicity, and the dose delivered as well as the
therapeutic efficacy can be accurately measured noninvasively over time. Much remains to be done before this
can be a clinical reality and many factors need to be optimized simultaneously for the best clinical outcome.
