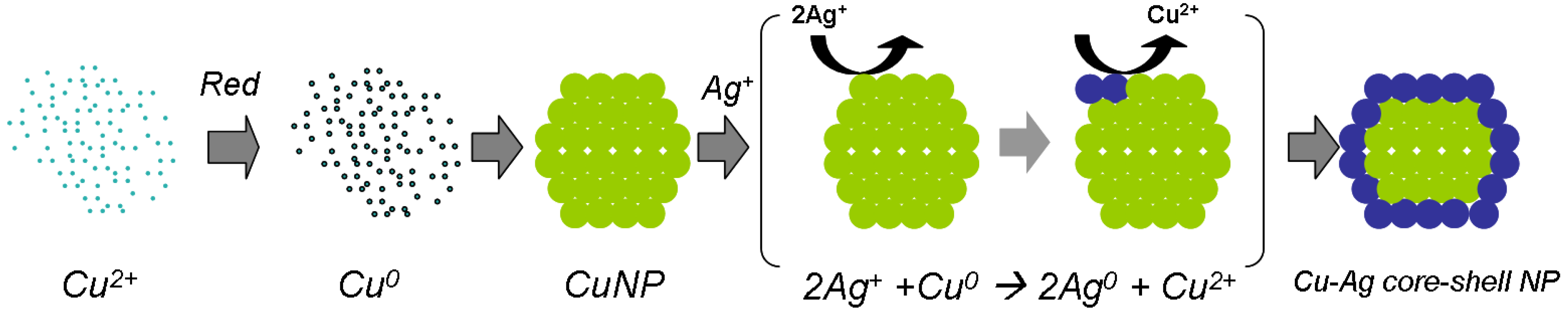
In the past few years, the synthesis of Cu nanoparticles has attracted much attention because of its huge
potential for replacing expensive nano silver inks utilized in conductive printing. A major problem in utilizing
these copper nanoparticles is their inherent tendency to oxidize in ambient conditions. Recently, there have
been several reports presenting various approaches which demonstrate that copper nanoparticles can resist
oxidation under ambient conditions, if they are coated by a proper protective layer. This layer may consist of an
organic polymer, alkene chains, amorphous carbon or graphenes, or inorganic materials such as silica, or an
inert metal. Such coated copper nanoparticles enable achieving high conductivities by direct printing of
conductive patterns. These approaches open new possibilities in printed electronics, for example by using
copper based inkjet inks to form various devices such as solar cells, Radio Frequency Identification (RFID) tags,
and electroluminescence devices. This paper provides a review on the synthesis of copper nanoparticles, mainly
by wet chemistry routes, and their utilization in printed electronics.
copper nanoparticles for printed electronics
At present most conductive inks are based on micron size Ag flakes. Ag powders (nano-sized particles) have
been commercially available for some years. Other metals used in inks are Cu and CuO. Also these
metal particles can be purchased in different particle sizes and morphologies (particles and nanowires) from
tens of nanometers to micron scale flakes. Ag possesses the highest electrical conductivity among metals (6,3 *
107 S/m) and is resistant to oxidation but has a high and fluctuating price (approximately 500 €/kg in January
2014). Typically nanoparticle based inks are considerably more expensive than inks based on flakes due to
higher price of nanoscale metal particles and special ink formulation.
Cu has almost the same conductivity (5,96*107 S/m) than Ag but is considerably less expensive (approximately 5
€/kg in January 2014). The challenge with Cu is its rapid oxidation in the air. Oxides are not conductive which
delimits Cu usage in printed electronics applications. Cu can be protected in ink formulation by using protecting
agents such as ligands. Low cost inks based on CuO contain agent which reduces oxide back to metallic copper
in photonic sintering process. Still Cu ink usage, if not protected or shielded in the application, is limited to only
certain, usually shorter life-time products. Advantages of nanoparticles over flakes are ability to reach same
conductivity with thinner and finer printed pattern with less metallic particles, ability to use non-contact printing
method with fragile substrates and ability to use lower sintering temperature which allows cheaper and
temperature sensitive substrates such as plastics and paper to be used. The main challenge is the manufacturing
cost and obtaining stable fluid dispersion in inks.
Printed electronics represent an emerging area of research that promises large markets due to the ability to
bypass traditional expensive and inflexible silicon-based electronics to fabricate a variety of devices on flexible
substrates using high-throughput printing approaches.
In the past few years, the synthesis of Cu nanoparticles has attracted much attention because of its huge
potential for replacing expensive nano silver inks utilized in conductive printing. A major problem in utilizing
these copper nanoparticles is their inherent tendency to oxidize in ambient conditions. Recently, there have
been several reports presenting various approaches which demonstrate that copper nanoparticles can resist
oxidation under ambient conditions, if they are coated by a proper protective layer. This layer may consist of an
organic polymer, alkene chains, amorphous carbon or graphenes, or inorganic materials such as silica, or an
inert metal. Such coated copper nanoparticles enable achieving high conductivities by direct printing of
conductive patterns. These approaches open new possibilities in printed electronics, for example by using
copper based inkjet inks to form various devices such as solar cells, Radio Frequency Identification (RFID) tags,
and electroluminescence devices.
