What are Lead Sulfide Quantum Dots (PbS QDs)?
Lead Sulfide Quantum Dots (PbS QDs) are a class of semiconductor nanoparticles that have attracted significant attention due to their unique optical and electronic properties, which are a result of their quantum size effects. These properties make PbS QDs suitable for a wide range of applications, particularly in optoelectronics, photovoltaics, and bioimaging. This article explores what PbS QDs are, their synthesis methods, properties, and key applications.
1. Introduction to Lead Sulfide Quantum Dots (PbS QDs)
Quantum dots (QDs) are nanometer-sized semiconductor particles that exhibit distinct properties compared to their bulk counterparts, primarily due to the effects of quantum confinement. In quantum dots, the motion of charge carriers (electrons and holes) is restricted to the nanometer-scale, leading to discrete energy levels. PbS QDs, composed of lead sulfide, are one of the most studied materials in the field of quantum dots, known for their tunable electronic and optical properties.
Lead sulfide is a narrow bandgap semiconductor, with its bulk bandgap around 0.41 eV. However, when reduced to the size of quantum dots, the bandgap can shift depending on the size of the particles, making them suitable for applications requiring a specific range of light absorption or emission.
2. Properties of PbS Quantum Dots
PbS quantum dots exhibit several fascinating properties that make them highly useful for various scientific and technological applications:
A. Size-Dependent Optical Properties
- Quantum Confinement: The most notable feature of PbS QDs is their size-dependent optical properties. As the size of the quantum dots decreases, the energy bandgap increases, shifting the absorption and emission spectra toward shorter wavelengths. This allows for the precise tuning of the absorption/emission properties by simply controlling the size of the QDs.
- Photoluminescence: PbS QDs show strong photoluminescence (PL) properties, meaning they can absorb light at a certain wavelength and re-emit it at a different wavelength. This emission is highly dependent on the size of the quantum dot, allowing for their use in color-tunable light sources.
B. Narrow Bandgap
PbS has a narrow bandgap, which makes it highly efficient for absorbing infrared (IR) light. PbS QDs can be tuned to absorb IR radiation by adjusting their size, which is beneficial for applications like infrared detection and solar energy harvesting.
C. High Surface Area and Reactivity
Like other quantum dots, PbS QDs have a high surface-to-volume ratio. This makes them highly reactive and suitable for applications involving surface interactions, such as in catalysis or sensing.
D. High Photocurrent and Charge Transport
PbS QDs can exhibit excellent charge transport properties, making them promising materials for optoelectronic devices like photodetectors, solar cells, and light-emitting devices.
3. Synthesis of PbS Quantum Dots
The synthesis of PbS quantum dots is a crucial aspect of determining their properties and performance in various applications. Several methods are commonly used to synthesize PbS QDs, including:
A. Colloidal Synthesis
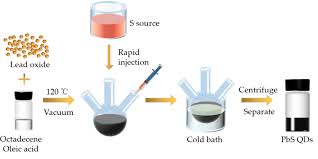
Colloidal synthesis is one of the most common and effective methods for producing PbS QDs. In this method, lead and sulfur precursors are mixed in a solvent at elevated temperatures. The reaction leads to the formation of PbS QDs, which are stabilized by surfactants or ligands to prevent aggregation. The size of the QDs can be controlled by adjusting reaction parameters such as temperature, precursor concentration, and reaction time.
B. Chemical Vapor Deposition (CVD)
CVD is another technique used to synthesize PbS QDs, particularly for applications requiring thin films or larger-scale production. In this method, lead and sulfur precursors are vaporized and then deposited onto a substrate, where they form PbS QDs.
C. Hydrothermal and Solvothermal Synthesis
These methods involve the use of water or other solvents under high temperature and pressure conditions to form PbS QDs. Hydrothermal and solvothermal synthesis are popular for creating high-quality quantum dots with precise control over size and morphology.
D. Electrochemical Synthesis
Electrochemical methods have been developed to fabricate PbS QDs by reducing lead ions at the electrode surface in the presence of sulfide ions. This method is less common but offers the advantage of producing QDs in aqueous or non-aqueous environments.
4. Applications of PbS Quantum Dots
The unique properties of PbS quantum dots have led to their exploration in various fields, including optoelectronics, energy, and biomedicine.
A. Photovoltaics and Solar Cells
PbS QDs have attracted significant attention for their potential in solar cells, particularly in the field of quantum dot-sensitized solar cells (QDSCs). The narrow bandgap of PbS QDs allows for the efficient absorption of infrared light, which is not typically absorbed by conventional semiconductor materials. Additionally, PbS QDs can be easily integrated into thin-film solar cells, potentially improving their efficiency.
- Quantum Dot Solar Cells (QDSCs): PbS QDs are used in QDSCs to improve light absorption across a broader spectrum, especially in the infrared range. By tuning the size of the QDs, they can absorb light over a wider range of wavelengths, enhancing overall efficiency.
B. Infrared Photodetectors
Due to their narrow bandgap and ability to absorb infrared light, PbS QDs are ideal for use in infrared photodetectors. These detectors are used in a variety of applications, such as thermal imaging, night vision, and remote sensing.
- Quantum Dot Photodetectors: PbS quantum dot photodetectors are highly sensitive to infrared radiation, making them suitable for applications that require detection of low-intensity infrared signals.
C. Light Emitting Diodes (LEDs)
PbS QDs can be used in LEDs, particularly for applications requiring narrow emission spectra or tunable colors. The size-dependent emission properties of PbS QDs make them ideal for color-tunable LED applications.
D. Bioimaging and Biosensing
PbS QDs are used in biological imaging and sensing due to their high fluorescence efficiency, stability, and size-tunable emission spectra. They can be conjugated with biomolecules, such as antibodies, for highly sensitive biological detection.
- Biological Applications: PbS QDs can be used as fluorescent probes for imaging cells, tissues, or specific molecules in vivo, offering a powerful tool for medical diagnostics and research.
E. Catalysis
The high surface area and tunable electronic properties of PbS QDs make them potential candidates for use in catalysis, particularly for photocatalytic reactions such as hydrogen production from water splitting or CO₂ reduction.
5. Challenges and Future Directions
While PbS QDs have demonstrated considerable promise in various applications, there are several challenges that need to be addressed:
- Toxicity and Environmental Concerns: Lead-based quantum dots, including PbS QDs, have raised concerns regarding their potential toxicity. The disposal and safe use of PbS QDs need to be carefully managed.
- Stability: The stability of PbS QDs, particularly in biological environments and under harsh operating conditions, can be a limiting factor.
- Scalability: Large-scale production of PbS QDs with consistent properties remains a challenge. Developing cost-effective and scalable methods for synthesis is crucial for the widespread adoption of PbS QDs in commercial applications.
Despite these challenges, ongoing research into PbS QDs is focused on improving their stability, environmental impact, and scalability, opening the door for further advancements in energy, electronics, and biomedical fields.
6. Conclusion
Lead Sulfide Quantum Dots (PbS QDs) are a fascinating class of semiconductor nanomaterials with a wide range of applications, from solar cells and photodetectors to bioimaging and catalysis. Their unique optical and electronic properties, such as size-tunable absorption and emission, make them highly versatile for next-generation technologies. As research continues, overcoming challenges related to stability, toxicity, and scalability will be essential to fully unlock the potential of PbS QDs for commercial applications and sustainable technological advancement.