Synthesis and Application Areas of Cerium Oxide (CeO₂)
Introduction
Cerium oxide (CeO₂), also known as ceria, is a versatile material with a wide range of applications across several industries. As one of the most important rare-earth oxides, cerium oxide exhibits unique chemical and physical properties, such as high thermal stability, oxygen storage capacity, and excellent catalytic activity. These properties make CeO₂ a critical material in various fields, including catalysis, energy storage, environmental remediation, and materials science.
This article delves into the synthesis methods of cerium oxide and explores its diverse application areas, highlighting its importance in both industrial and environmental technologies.
Synthesis of Cerium Oxide (CeO₂)
Cerium oxide can be synthesized through several methods, each of which affects the material’s physical and chemical properties, such as particle size, crystallinity, and surface area. Some of the most common synthesis techniques include:
1. Precipitation Method
- Process: This is one of the most widely used methods for synthesizing CeO₂. It involves adding a precipitating agent (such as ammonium hydroxide) to a cerium salt solution (e.g., cerium nitrate or cerium chloride), causing cerium hydroxide to form. The hydroxide is then calcined (heated at high temperatures) to produce cerium oxide.
- Advantages: Simple, cost-effective, and scalable for large production.
- Applications: The precipitation method is commonly used for the synthesis of CeO₂ nanoparticles with high surface area and fine particle distribution, making it ideal for catalytic and energy applications.
2. Sol-Gel Method
- Process: The sol-gel method involves the transition of a solution (sol) into a gel, followed by drying and calcination to obtain CeO₂. In this method, cerium precursors such as cerium nitrate are mixed with a gelling agent, like citric acid, to form a sol. The gel is then dried and calcined at high temperatures to produce cerium oxide.
- Advantages: Allows for precise control over the morphology and size of the cerium oxide particles, resulting in high-purity material.
- Applications: The sol-gel method is used to produce CeO₂ for applications that require high surface area and homogeneity, such as in catalysis and energy storage.
3. Hydrothermal Synthesis
- Process: In this method, CeO₂ is synthesized by reacting cerium salts with a solvent under high temperature and pressure conditions. The hydrothermal environment helps control particle growth, yielding uniform CeO₂ crystals.
- Advantages: High purity, control over particle size, and the ability to synthesize CeO₂ in different forms (nanoparticles, nanorods, etc.).
- Applications: Used for creating high-quality CeO₂ for advanced technological applications like catalysis, sensors, and fuel cells.
4. Solid-State Reaction
- Process: In solid-state synthesis, CeO₂ is obtained by directly heating a mixture of cerium salts (such as cerium carbonate or cerium nitrate) and a solid base (e.g., sodium hydroxide) at elevated temperatures. This method is relatively simple and cost-effective.
- Advantages: Suitable for large-scale production.
- Applications: Primarily used for bulk production of cerium oxide for industrial applications such as polishing and catalyst supports.
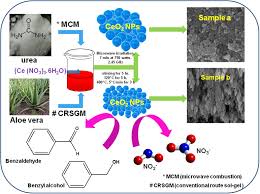
5. Microwave-Assisted Synthesis
- Process: This is a relatively newer method where microwave radiation is used to heat the precursors rapidly, causing cerium oxide to form in a shorter time than traditional heating methods.
- Advantages: Faster synthesis with higher energy efficiency and reduced formation of unwanted by-products.
- Applications: Suitable for producing CeO₂ for applications where rapid processing and precise control over the material properties are required.
Application Areas of Cerium Oxide (CeO₂)
Cerium oxide’s remarkable chemical and physical properties make it suitable for a wide array of applications, spanning multiple industries such as energy, environmental remediation, electronics, and materials science.
1. Catalysis and Automotive Emissions Control
- Catalytic Converters: One of the most important applications of CeO₂ is in catalytic converters for the automotive industry. Cerium oxide serves as an oxygen storage component in three-way catalysts, which are used to reduce harmful emissions like carbon monoxide (CO), nitrogen oxides (NOₓ), and hydrocarbons (HC) from vehicle exhaust.
- Oxygen Storage: CeO₂ has the ability to store and release oxygen, a property that helps in reducing NOₓ emissions, especially during periods of high exhaust temperatures when oxygen is needed to oxidize NOₓ to nitrogen and oxygen.
- Environmental Impact: By facilitating more efficient combustion and reducing emissions, CeO₂ contributes to cleaner air quality.
2. Fuel Cells and Energy Storage
- Solid Oxide Fuel Cells (SOFCs): CeO₂ is used as a component in the anode of Solid Oxide Fuel Cells, which are a key technology for clean energy generation. Its ability to conduct oxygen ions at high temperatures makes it an excellent candidate for fuel cell applications, improving efficiency and stability.
- Oxygen Storage and Release: CeO₂ is a highly efficient material for storing and releasing oxygen, which is critical in applications like oxygen pumps and rechargeable batteries. In energy storage systems, cerium oxide’s oxygen storage capacity allows for the efficient conversion of energy, making it a potential material for next-generation energy storage devices.
3. Environmental Remediation and Pollution Control
- Water Treatment: Cerium oxide has been explored for its ability to remove heavy metals, such as lead and mercury, from contaminated water. CeO₂ can adsorb and neutralize harmful pollutants, making it a valuable material for water purification processes.
- Air Purification: In addition to its use in automotive catalytic converters, CeO₂ is also used in air filtration systems to reduce particulate matter and pollutants in the air, helping mitigate environmental pollution.
4. Nanomaterials for Electronics and Sensors
- Sensors: Cerium oxide nanoparticles have been explored for use in gas sensors, particularly for detecting harmful gases like carbon monoxide and nitrogen dioxide. Due to their high surface area and excellent catalytic properties, CeO₂ nanoparticles are ideal for such sensing applications.
- Semiconductor Materials: Cerium oxide’s electronic properties make it useful in the development of semiconductor materials, particularly in electronic devices that require high efficiency, such as light-emitting diodes (LEDs) and solar cells.
5. Polishing and Material Science
- Polishing Agents: CeO₂ is widely used as a polishing agent in industries such as optical lens polishing, glass polishing, and the semiconductor industry. The fine powder of CeO₂ is particularly effective in polishing and smoothing surfaces, making it a key component in the production of high-precision optical devices.
- Ceramic and Glass Industries: CeO₂ is used as a fluxing agent in the production of specialized ceramic materials and glass. It helps improve the optical properties of glass, especially in fiber optics and precision lenses used in telecommunications and scientific instruments.
6. Pharmaceuticals and Biomedical Applications
- Drug Delivery: Due to its biocompatibility and ability to be functionalized, cerium oxide nanoparticles are being explored for use in drug delivery systems. They are particularly useful for targeting specific cells and tissues, providing controlled drug release.
- Antioxidant Properties: Cerium oxide nanoparticles possess antioxidant activity and can be used in the development of novel therapeutics for diseases associated with oxidative stress, such as cancer, neurodegenerative diseases, and diabetes.
7. Solar Energy Applications
- Solar Cells: Cerium oxide is used in solar cells as a component that can improve the efficiency of light absorption and conversion. CeO₂ can be incorporated into photovoltaic devices to enhance their performance under varying light conditions.
8. Hydrogen Production
- Hydrogen Generation: CeO₂ has been investigated for its role in hydrogen production, particularly in water splitting reactions. As a catalyst, CeO₂ can facilitate the production of hydrogen gas from water, a key step in the development of clean energy technologies such as hydrogen fuel cells.
Conclusion
Cerium oxide (CeO₂) is a material with vast potential across numerous industries due to its unique properties, such as high oxygen storage capacity, excellent catalytic activity, and thermal stability. From automotive emissions control to energy storage, pharmaceuticals, and water treatment, CeO₂ continues to make significant contributions to both environmental and industrial applications. As research progresses, new applications are being explored, further expanding its role in sustainable technologies and advanced material science.
