Revealing the PVDF Binder Performance for Li-ion Batteries
In the world of lithium-ion batteries (Li-ion), polyvinylidene fluoride (PVDF) plays a crucial role as a binder material. The binder in Li-ion batteries is responsible for holding together the active material particles, ensuring that the electrode remains stable throughout the battery’s charging and discharging cycles. The performance of the PVDF binder directly impacts the overall efficiency, capacity, and lifetime of Li-ion batteries. This article explores the properties, performance, and applications of PVDF binders in the battery industry, highlighting their significance in improving the functionality of Li-ion batteries.
What is PVDF (Polyvinylidene Fluoride)?
Polyvinylidene fluoride (PVDF) is a high-performance fluoropolymer that is widely used in various industries due to its exceptional chemical resistance, mechanical properties, and electrochemical stability. In Li-ion batteries, PVDF is commonly used as a binder material in the electrode fabrication process.
PVDF’s key characteristics that make it ideal for use in Li-ion batteries include:
- High chemical resistance, which prevents degradation from exposure to electrolytes.
- Electrochemical stability, ensuring that PVDF does not interfere with the battery’s performance.
- Mechanical strength, helping to maintain the integrity of the electrode during repeated charge and discharge cycles.
Role of PVDF Binder in Li-ion Batteries
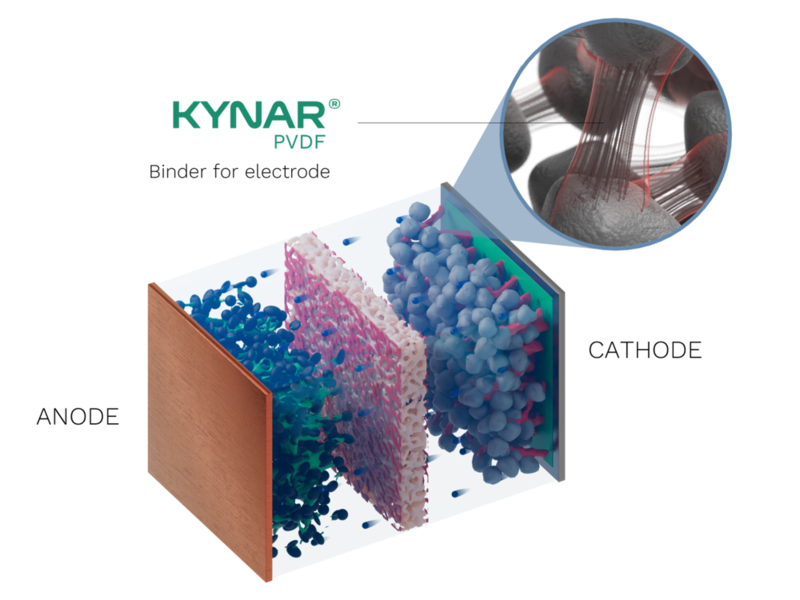
The primary function of the PVDF binder in Li-ion batteries is to hold the active materials (such as lithium cobalt oxide, graphite, or silicon-based compounds) together in the electrode. This binding effect is vital to maintaining the structural integrity of the electrode, ensuring the electrical conductivity and cycling stability of the battery.
The binder also plays a crucial role in:
- Ensuring adhesion between the active materials and the current collectors (usually made of copper or aluminum).
- Facilitating the formation of stable electrode layers, contributing to the overall energy density of the battery.
- Maintaining battery efficiency over multiple charge-discharge cycles, as it helps prevent material separation during cycling.
Performance Factors of PVDF Binder
The performance of the PVDF binder in Li-ion batteries can be influenced by various factors such as molecular structure, concentration, and the dispersion of the binder in the electrode slurry. The following factors are crucial for maximizing the binder’s performance:
- Molecular Weight and Polymer Chain Structure:
- The molecular weight of the PVDF binder directly impacts its mechanical properties. Higher molecular weight PVDF can enhance the adhesion strength between the active materials and the current collectors, leading to better cycle stability.
- Solubility in Organic Solvents:
- PVDF is typically dissolved in organic solvents like N-Methyl-2-pyrrolidone (NMP) before being mixed with active materials. The solubility of PVDF in these solvents is critical for achieving a uniform slurry, ensuring good dispersion of the binder within the electrode.
- Binder-to-Active Material Ratio:
- The ratio of PVDF binder to active materials is an important factor. Too much binder can increase the resistance and decrease the energy density, while too little binder may result in poor adhesion and reduced cycling stability. The optimal ratio needs to balance structural integrity and electrical conductivity.
- Electrochemical Stability:
- PVDF’s ability to withstand the electrochemical environment within the battery, particularly during the charge-discharge cycles, is critical for the battery’s longevity and capacity retention. PVDF binder’s stability ensures that it does not decompose or interact with the electrolyte, maintaining the battery’s overall performance.
- Conductivity and Ion Transport:
- While PVDF is an excellent binder, it is not conductive. To improve ion transport and overall conductivity, PVDF is often mixed with conductive additives, such as carbon black or carbon nanotubes. This enhances the battery’s overall conductivity, improving efficiency.
Benefits of PVDF Binder in Li-ion Batteries
- Improved Cycle Life:
- PVDF binder plays a key role in increasing the cycle life of Li-ion batteries. By holding the electrode materials together during repeated charge-discharge cycles, it helps to prevent particle loss and electrode degradation, which can lead to reduced capacity over time.
- Enhanced Energy Density:
- The mechanical strength of the PVDF binder allows for a denser electrode structure, increasing the overall energy density of the battery. This is especially important for applications in electric vehicles and portable electronics, where high energy density is critical.
- Excellent Thermal Stability:
- PVDF has a high thermal stability, meaning it can withstand the heat generation that occurs during the operation of Li-ion batteries. This contributes to the safety and reliability of the battery under high temperature conditions.
- Improved Conductivity:
- As mentioned, the addition of conductive fillers like carbon black to the PVDF binder enhances the electrical conductivity of the electrodes. This leads to better charge/discharge rates and overall battery performance.
Challenges with PVDF Binder
While PVDF offers excellent performance in many ways, there are some challenges associated with its use:
- Environmental Impact: PVDF is a fluoropolymer, which can pose environmental concerns during disposal or incineration. Research is ongoing into more sustainable and eco-friendly alternatives for Li-ion battery binders.
- Solvent Usage: The organic solvent, NMP, used to dissolve PVDF is toxic and harmful to both human health and the environment. Efforts are being made to reduce or eliminate the use of NMP in battery production by exploring water-based solutions.
- Cost: PVDF is relatively expensive compared to some alternative binders, which could affect the overall cost-effectiveness of large-scale battery production.
Emerging Alternatives to PVDF Binder
Given the challenges with PVDF, there has been increasing research into alternative binders that could provide similar or even better performance while reducing environmental and cost concerns. Some alternatives include:
- Polyacrylic acid (PAA): A water-soluble polymer that offers good adhesion properties and could reduce the environmental impact associated with PVDF.
- Carboxymethyl cellulose (CMC): A biopolymer that is gaining attention as an eco-friendly alternative with good electrochemical performance.
Conclusion
The PVDF binder plays a pivotal role in the performance of Li-ion batteries, ensuring the structural integrity of the electrodes, improving cycle life, and contributing to high energy density. Despite challenges related to environmental impact and cost, PVDF remains a popular choice due to its excellent electrochemical stability and mechanical properties. With ongoing research into alternative binders, the future of Li-ion batteries looks promising, offering improved performance and sustainability for the growing battery industry. Understanding the performance of PVDF binders is crucial for advancing battery technology and addressing the increasing demand for efficient and long-lasting energy storage solutions.