Iron-Air Batteries: The Ultimate Guide
Iron-air batteries are an emerging technology that is gaining attention for its potential to provide long-duration energy storage with high efficiency and cost-effectiveness. These batteries, also known as iron-air flow batteries, offer a promising alternative to traditional lithium-ion batteries, especially in applications that require large-scale energy storage systems, such as renewable energy integration and grid storage. In this ultimate guide, we’ll explore the fundamentals of iron-air batteries, how they work, their advantages, challenges, and future potential.
1. What Are Iron-Air Batteries?
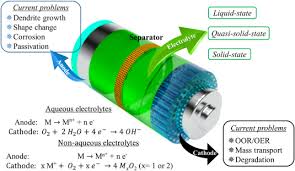
Iron-air batteries are a type of metal-air battery that uses iron (Fe) as the anode and oxygen (O₂) from the air as the cathode. The concept behind metal-air batteries involves an electrochemical reaction where oxygen is reduced at the cathode, and the metal is oxidized at the anode. Iron-air batteries are similar in principle to lithium-air batteries, but they use iron as the primary metal for energy storage, which makes them more abundant and cost-effective.
Iron-air batteries typically consist of the following components:
- Anode (Iron): Iron serves as the energy storage medium, where the oxidation process takes place.
- Cathode (Air/Oxygen): Oxygen from the surrounding air is reduced at the cathode, enabling the battery to discharge and release energy.
- Electrolyte: The electrolyte conducts ions between the anode and cathode, facilitating the flow of electric charge during discharge and charge cycles.
These batteries are mainly designed for long-duration energy storage applications, where they can charge and discharge over extended periods, making them ideal for storing renewable energy from sources like solar and wind power.
2. How Do Iron-Air Batteries Work?
The operation of an iron-air battery involves an electrochemical reaction between iron and oxygen. The fundamental process can be broken down into the following stages:
Discharge Process:
- Oxidation of Iron at the Anode: When the battery is discharging, iron at the anode reacts with hydroxide ions from the electrolyte. The iron loses electrons and forms iron oxide (Fe₂O₃), which releases energy in the form of electricity.
2Fe+6OH−→Fe2O3+3H2O+6e−2Fe + 6OH^- \rightarrow Fe_2O_3 + 3H_2O + 6e^-2Fe+6OH−→Fe2O3+3H2O+6e−
- Reduction of Oxygen at the Cathode: At the cathode, oxygen from the air is reduced by gaining electrons. The reduced oxygen forms hydroxide ions (OH⁻), which then migrate to the anode to complete the circuit.
O2+4e−+2H2O→4OH−O_2 + 4e^- + 2H_2O \rightarrow 4OH^-O2+4e−+2H2O→4OH−
The flow of electrons between the anode and cathode generates electric power.
Charging Process:
During charging, the reactions reverse:
- Reduction of Iron Oxide at the Anode: The iron oxide at the anode is reduced back to metallic iron, absorbing electrical energy in the process.
- Release of Oxygen at the Cathode: Oxygen from the air is released at the cathode, completing the charge cycle.
3. Advantages of Iron-Air Batteries
Iron-air batteries offer several key advantages, which make them a strong contender in the energy storage market:
A. Cost-Effectiveness:
Iron is one of the most abundant and inexpensive metals on Earth, making iron-air batteries a cost-effective alternative to traditional battery technologies like lithium-ion. The raw materials for iron-air batteries are far cheaper than lithium, cobalt, and other rare elements used in lithium-ion batteries.
B. Long Duration Energy Storage:
Iron-air batteries are known for their ability to store energy over long durations. This is particularly useful for applications like grid energy storage, where electricity produced from renewable sources like solar and wind needs to be stored for later use, sometimes over long periods.
C. High Energy Density:
Iron-air batteries can offer higher energy density compared to traditional lead-acid and other flow batteries, making them suitable for larger-scale energy storage.
D. Environmental Benefits:
Iron-air batteries are more environmentally friendly due to the use of abundant and non-toxic materials, unlike lithium-ion batteries that rely on materials that can have a significant environmental footprint. Additionally, iron is easily recyclable, which adds to the sustainability of iron-air batteries.
E. Scalability:
Due to the availability of inexpensive materials and relatively simple construction, iron-air batteries can be scaled up for large-scale applications, such as grid storage, providing an affordable solution for storing renewable energy.
4. Challenges of Iron-Air Batteries
Despite their potential, iron-air batteries also face several technical challenges that must be addressed before they can be widely adopted:
A. Limited Cycle Life:
Iron-air batteries can suffer from limited cycle life due to the formation of iron oxide (rust) during discharge. This can lead to a decrease in the battery’s efficiency over time, requiring periodic maintenance or replacement of the anode material.
B. Efficiency Issues:
The efficiency of iron-air batteries during charging and discharging is currently lower than that of lithium-ion batteries. This is due to factors such as the slower kinetics of the oxygen reduction and evolution reactions, which affect overall performance.
C. Slow Charge Times:
Iron-air batteries can take longer to charge compared to lithium-ion batteries, which can limit their use in applications where rapid charging is required.
D. Air Contamination:
Since iron-air batteries rely on oxygen from the air, they can be affected by environmental factors like humidity, temperature, and the presence of other gases. These factors can impact the efficiency and lifespan of the battery.
E. Complex Electrolyte Systems:
The electrolyte systems in iron-air batteries need to be carefully designed to ensure good conductivity and long-term stability. Finding the right electrolyte is crucial to improving the battery’s performance and longevity.
5. Applications of Iron-Air Batteries
Iron-air batteries are still in the development phase, but they show great promise in several key applications:
A. Grid Energy Storage:
Iron-air batteries are particularly well-suited for grid-scale energy storage, where they can store energy produced by renewable sources like wind and solar power for use during periods of low energy production or high demand.
B. Backup Power Systems:
These batteries can be used in backup power systems for homes, businesses, and critical infrastructure, providing long-duration backup power in case of grid failures.
C. Electric Vehicles (EVs):
While not yet widely used in electric vehicles, the high energy density and low cost of iron-air batteries could make them an attractive option for EVs in the future, offering longer driving ranges and cheaper batteries compared to lithium-ion.
D. Remote and Off-Grid Applications:
Iron-air batteries could also be used in off-grid or remote locations where reliable, long-duration energy storage is needed, especially in areas where solar and wind power are abundant.
6. The Future of Iron-Air Batteries
The future of iron-air batteries looks promising as research continues to improve their efficiency, lifespan, and performance. Innovations in electrode materials, electrolytes, and battery design could help overcome the current challenges, making iron-air batteries a competitive alternative to traditional energy storage solutions. As the demand for renewable energy storage grows, iron-air batteries could play a crucial role in reducing costs and improving the sustainability of energy systems worldwide.
7. Conclusion
Iron-air batteries are an exciting development in the field of energy storage. With their cost-effectiveness, environmental benefits, and potential for long-duration energy storage, they offer a promising solution for large-scale applications like grid storage and backup power systems. Although there are still challenges to overcome, including improving efficiency and cycle life, ongoing research and advancements in materials and technology could help iron-air batteries become a key player in the future of sustainable energy storage.