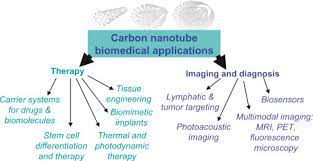
Super Activated Carbon Nanotubes Drug Delivery
CNTs can be used as drug carriers to treat tumours. The efficacy of anticancer drugs used alone is restrained
not only by their systemic toxicity and narrow therapeutic window but also by drug resistance and limited
cellular penetration. Because CNTs can easily across the cytoplasm membrane and nuclear membrane,
anticancer drug transported by this vehicle will be liberated in situ with intact concentration and consequently,
its action in the tumour cell will be higher than that administered alone by traditional therapy. Thus, the
development of efficient delivery systems with the ability to enhance cellular uptake of existing potent drugs is
needed. The high aspect ratio of carbon nanotubes for biomedical applications offers great advantages over
the existing delivery vectors, because the high surface area provides multiple attachment sites for drugs. Many
anticancer drugs have been conjugated with functionalized CNTs and successfully tested in vitro and in vivo
such as epirubicin, doxorubicin, cisplatin, methotrexate, quercetin, and Paclitaxel. For avoiding the harmful
effect of anticancer drug on healthy organs and cells, has linked epirubicin with a magnetic CNTs complex
obtained by fixing a layer of magnetite (Fe3O4) nanoparticles on the surface of the nanotubes with necklace-like
type and on the tips of shortened MWCNTs. The used the epirubicin magnetic CNTs complex for lymphatic
tumour targeting. Such a system can be guided by an externally placed magnet to target regional lymphatic
nodes. Chemotherapeutic agents can be bound to a complex formed by CNT and antibody against antigen over
expressed on the cancerous cell surface. The attraction of antigen-antibody, the CNTs can be taken up by the
tumour cell only before the anticancer drug is cleaved off CNTs; thus, targeting delivery is realized.
Carbon Nanotubes for Gene Therapy by DNA Delivery
carbon nanotubes for biomedical applications is an approach to correct a defective gene which is the cause of
some chronic or hereditary diseases by introducing DNA molecule into the cell nucleus. Some delivery systems
for DNA transfer include liposome’s, cationic lipids and nanoparticles such as CNTs recently discovered. When
bound to SWCNTs, DNA probes are protected from enzymatic cleavage and interference from nucleic acid
binding proteins, consequently, DNA-SWCNT complex exhibits superior bio stability and increases self-delivery
capability of DNA in comparison to DNA used alone. Indeed, stable complexes between plasmid DNA and
cationic CNTs have demonstrated the enhancement of gene therapeutic capacity compared with naked DNA.
CNTs conjugated with DNA were found to release DNA before it was destroyed by cells defence system,
boosting transfect ion significantly. The use of CNTs as gene therapy vectors has shown that these engineered
structures can effectively transport the genes inside mammalian cells and keep them intact because the CNTgene complex has conserved the ability to express proteins. Pantarotto and co-workers have developed novel functionalized SWCNT-DNA complexes and reported high DNA expression compared with naked DNA.
carbon nanotubes for biomedical applications
Cell and organ transplantation and of CNT chemistry in recent years have contributed to the sustained
development of CNT-based tissue engineering and regenerative medicine. Carbon nanotubes may be the best
tissue engineering candidate among numerous other materials such as natural and synthetic polymers for
tissue scaffolds since this nanomaterial is biocompatible, resistant to biodegradation, and can be
functionalized with biomolecules for enhancing the organ regeneration. In this field, CNTs can be used as
additives to reinforce the mechanical strength of tissue scaffolding and conductivity by incorporating with the
host’s body. Other tissue engineering applications of CNTs concerning cell tracking and labeling, sensing cellular
behaviour, and enhancing tissue matrices are also studied recently. For example, it has been reported that CNTs
can effectively enhance bone tissue regenerations in mice and neurogenic cell differentiation by embryonic stem
cells in vitro.
carbon nanotubes for biomedical applications
A biosensor is an analytical device, used for the detection of an analyse that combines a biological component
with a physicochemical detector. The use of CNTs in bio sensing nanotechnology is recent and represents a most
exciting application area for therapeutic monitoring and in vitro and in vivo diagnostics. For example, coupled
CNTs with glucose-oxidise biosensors for blood sugar control in diabetic patient with higher accuracy and
simpler manipulation than biosensors used alone. Other CNT-enzyme biosensors such as CNT-based
dehydrogenase biosensors or peroxidase and catalyse biosensors have also been developed for different
therapeutic monitoring and diagnostics. Electrical detection of DNA, the assay sensitivity was higher with
alkaline phosphatase (ALP) enzyme linked to CNTs than with ALP alone. The sensitivity of the assay using
SWCNT-DNA sensor obtained by integration of SWCNTs with single-strand DNAs (ssDNA) was considerably
higher than traditional fluorescent and hybridization assays. This CNT-biosensor-linked assay can be modified
for antigen detection by using specific antibody-antigen recognition. Thus, it could provide a fast and simple
solution for molecular diagnosis in pathologies where molecular markers exist, such as DNA or protein. CNTs
have been assayed to detect some organophosphoric pesticides by using acetylcholine esterase immobilized on
CNT surface with electrochemical detection. Owing to their length scale and unique structure, the use of CNTs as
biosensor vehicle is highly recommended to develop sensitive techniques for diagnostics and analyses from the
laboratory to the clinic.
Carbon Nanotubes for Infection Therapy
carbon nanotubes for biomedical applications Because of the resistance of infectious agents against numerous
antiviral, antibacterial drugs or due to certain vaccine inefficacy in the body, CNTs have been assayed to resolve
these problems. Functionalized CNTs have been demonstrated to be able to act as carriers for antimicrobial
agents such as the antifungal amphotericin. CNTs can attach covalently to amphotericin B and transport it into
mammalian cells. This conjugate has reduced the antifungal toxicity about 40% as compared to the free drug.
Our group has successfully combined an antimicrobial agent Pazufloxacin mediate with amino-MWCNT with high adsorption and will be applied to experimental assays for infection treatment. Functionalized CNTs can also act as vaccine delivery procedures. The linkage of a bacterial or viral antigen with CNTs permits of keeping
intact antigen conformation, thereby, inducing antibody response with the right specificity. The fixation of
functionalized CNTs with B and T cell peptide epitomes can generate a multivalent system able to induce a
strong immune response, thereby becoming a good candidate for vaccine delivery. Thus, functionalized CNTs
can act a good carrier system for the delivery of candidate vaccine antigens. Besides, CNTs themselves might
have antimicrobial activity since bacteria may be adsorbed onto the surfaces of CNTs, such as the case of E.
coli. The antibacterial effect was attributed to carbon nanotube-induced oxidation of the intracellular
antioxidant glutathione, resulting in increased oxidative stress on the bacterial cells and eventual cell death.
By Antitumor Immunotherapy
carbon nanotubes for biomedical applications used as carriers can be effectively applied in antitumor
immunotherapy. This therapeutic consists of stimulating the patient’s immune system to attack the malignant
tumour cells. This stimulation can be achieved by the administration of a cancer vaccine or a therapeutic
antibody as drug. Some authors have validated the use of carbon nanotubes for biomedical applications as
vaccine delivery tools. The conjugate of MWCNTs and tumour lysine protein (tumour cell vaccine) can
considerably and specifically enhance the efficacy of antitumor immunotherapy in a mouse bearing the H22 liver
tumour. In vitro, the conjugate of CNTs and tumour immunogens can act as natural antigen presenting cells
(such as mature dendrite cells) by bringing tumour antigens to immune effectors’ T cells; this action is due to
the high avidity of antigen on the surface and the negative charge.
