Selenium Dioxide Nanoparticles and Their Applications
Selenium dioxide (SeO₂) is a compound of selenium that exhibits distinct properties, including high oxidation states and unique chemical reactivity. As a result, selenium dioxide nanoparticles (SeO₂ NPs) are gaining significant attention in various fields of research, owing to their unique properties that differ from bulk selenium dioxide. Selenium dioxide nanoparticles combine the well-known benefits of selenium with the enhanced characteristics provided by the nanoscale dimensions. These nanoparticles have a wide range of applications across industries such as pharmaceuticals, electronics, energy storage, environmental remediation, and catalysis.
In this article, we will explore the properties of selenium dioxide nanoparticles, how they are synthesized, and the diverse industrial applications they are poised to impact.
What Are Selenium Dioxide Nanoparticles?
Selenium dioxide (SeO₂) is an inorganic compound that typically exists in the form of a colorless crystalline solid. When reduced to the nanoscale, selenium dioxide exhibits several unique characteristics due to the high surface area, high reactivity, and quantum effects that are inherent in nanoparticles. Selenium dioxide nanoparticles (SeO₂ NPs) have dimensions typically ranging from 1 nm to 100 nm and possess high surface energy, making them highly reactive and able to interact with a variety of substrates in ways that bulk SeO₂ cannot.
Due to their size-dependent properties—which differ from their bulk counterpart—selenium dioxide nanoparticles are used in various applications where their reactivity, optical properties, and electronic characteristics can be leveraged to provide new technological advancements.
Properties of Selenium Dioxide Nanoparticles
Selenium dioxide nanoparticles exhibit several important properties that make them suitable for diverse applications:
1. High Surface Area and Reactivity
- Due to their nanoscale size, SeO₂ NPs have a high surface-to-volume ratio, which increases their reactivity compared to bulk SeO₂. This makes them valuable in chemical reactions, catalysis, and sensor applications.
2. Semiconductor and Optical Properties
- Selenium compounds, including selenium dioxide nanoparticles, possess semiconductor-like properties. SeO₂ NPs have a band gap that allows them to be used in electronic and optical devices. These properties make SeO₂ nanoparticles potential candidates for use in photovoltaics, light-emitting devices, and sensors.
3. Antimicrobial and Antioxidant Properties
- Selenium is known for its antioxidant properties, which make selenium dioxide nanoparticles useful in biomedical applications, particularly for combating oxidative stress and as potential antimicrobial agents against various pathogens.
4. Catalytic Activity
- Selenium dioxide nanoparticles exhibit high catalytic efficiency due to their high surface area and ability to interact with a variety of reactants. They are used in organic synthesis and industrial catalysis.
5. Toxicity
- While selenium is an essential trace element in the human body at low concentrations, selenium dioxide nanoparticles can be toxic at high doses. However, their controlled use in applications such as drug delivery or catalysis offers new opportunities to explore selenium’s benefits without excessive toxicity.
Synthesis of Selenium Dioxide Nanoparticles
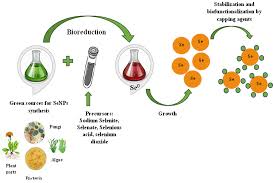
The synthesis of selenium dioxide nanoparticles involves various methods, each tailored to the desired size, shape, and functional properties of the nanoparticles. Common techniques include:
1. Chemical Vapor Deposition (CVD)
- In this method, selenium vapor reacts with oxygen or other oxidants at high temperatures to form selenium dioxide. This is one of the most efficient methods for producing uniform SeO₂ nanoparticles with a controlled size and distribution.
2. Sol-Gel Method
- The sol-gel process involves the hydrolysis and condensation of selenium precursors in a liquid phase to form selenium dioxide nanoparticles. This method offers a cost-effective approach to synthesizing SeO₂ nanoparticles and allows for the preparation of nanoparticles with controlled morphologies.
3. Hydrothermal Synthesis
- Hydrothermal methods use high-temperature, high-pressure conditions to facilitate the formation of selenium dioxide nanoparticles from selenium-containing precursors. This technique allows for the precise control of particle size, shape, and crystallinity.
4. Electrochemical Synthesis
- Electrochemical deposition methods use electrolysis of selenium salts in an electrolyte solution to deposit selenium dioxide nanoparticles. This method is useful for creating thin films or nano-sized coatings on conductive surfaces.
5. Laser Ablation
- Laser ablation in liquid is another method used for producing selenium dioxide nanoparticles. This involves the use of laser energy to break down selenium sources into nanoparticles that can be suspended in a liquid medium.
Applications of Selenium Dioxide Nanoparticles
Selenium dioxide nanoparticles are increasingly finding applications in a range of industries due to their unique properties. Some of the key industrial applications of SeO₂ nanoparticles include:
1. Catalysis and Chemical Synthesis
- Selenium dioxide nanoparticles are used as catalysts in organic synthesis, particularly in reactions like oxidation, reduction, and hydrogenation. Their ability to facilitate chemical reactions with high efficiency makes them valuable in both industrial and lab-scale chemical processes.
- Selenium dioxide is commonly used as a reagent in the epoxidation of alkenes and the synthesis of selenides. In nanoparticle form, SeO₂ demonstrates significantly improved reactivity compared to its bulk counterpart, thus accelerating reaction rates.
2. Electronics and Optoelectronics
- SeO₂ nanoparticles, with their semiconducting properties, have potential applications in electronic devices. Due to their optical properties, they can be used in the fabrication of photovoltaic cells, solar cells, and light-emitting diodes (LEDs).
- Selenium compounds, when incorporated into thin-film transistors (TFTs), can enhance device performance, making SeO₂ nanoparticles an important candidate for the electronics industry.
3. Energy Storage and Conversion
- Selenium dioxide nanoparticles can be used in energy storage systems, such as batteries and supercapacitors, due to their electrochemical properties. The high surface area of SeO₂ NPs enhances charge-discharge cycles and increases the storage capacity of energy devices.
- They are also used in photoelectrochemical applications, such as solar energy conversion, where SeO₂ nanoparticles help improve the efficiency of solar cells by enhancing light absorption and electron mobility.
4. Environmental Remediation
- Selenium dioxide nanoparticles are used in environmental cleanup technologies due to their ability to react with and degrade pollutants. They can be employed in water treatment applications to remove heavy metals and organic contaminants from water and wastewater.
- The high reactivity of SeO₂ NPs makes them excellent candidates for environmental catalysis, helping in the breakdown of organic pollutants and toxins in industrial wastewater treatment.
5. Biomedical and Pharmaceutical Applications
- Selenium is known for its antioxidant properties, which can be leveraged in drug delivery and biomedical applications. Selenium dioxide nanoparticles are being explored for use in targeted drug delivery systems, particularly for delivering antioxidants or anticancer agents to specific tissues or cells.
- Additionally, SeO₂ nanoparticles have shown promise in biosensor applications, particularly in detecting biomarkers or pathogens in biological samples.
6. Antimicrobial and Antiviral Agents
- Selenium dioxide nanoparticles are being researched for their antimicrobial properties. They can be used to combat bacterial and viral infections by disrupting the cell membranes of microbes or by producing reactive oxygen species (ROS) that inhibit pathogen growth.
- These properties make SeO₂ nanoparticles a promising alternative for the development of antibacterial coatings, wound dressings, and antiviral agents.
Challenges and Future Prospects
While selenium dioxide nanoparticles show considerable potential across various applications, there are challenges that must be addressed for their widespread adoption:
- Toxicity and Biocompatibility: While selenium is an essential trace element, the toxicity of selenium dioxide nanoparticles at high concentrations needs to be carefully managed, especially in biomedical applications. Ensuring their biocompatibility is crucial to making them viable for use in medicine and pharmaceuticals.
- Scalability: Many of the synthesis techniques for selenium dioxide nanoparticles are still in the research phase or are limited to small-scale production. Developing methods for mass production that are cost-effective and environmentally friendly will be key to their commercial viability.
- Environmental Impact: Like other nanoparticles, the environmental impact of selenium dioxide nanoparticles needs to be thoroughly assessed to ensure that they do not pose risks to ecosystems or human health when disposed of improperly.
Conclusion
Selenium dioxide nanoparticles offer a wide array of unique properties that make them valuable for a diverse range of industrial and technological applications. From catalysis and energy storage to environmental remediation and biomedical applications, SeO₂ nanoparticles hold immense promise in advancing existing technologies and opening up new possibilities in several sectors. With continued research into their synthesis, toxicity, and scalability, selenium dioxide nanoparticles are set to play a pivotal role in the development of next-generation materials for energy, healthcare, and environmental technologies.
