Al2O3 Nanoparticles: Properties, Synthesis, and Applications
Aluminum oxide (Al2O3) nanoparticles, commonly referred to as alumina nanoparticles, are a highly versatile material with remarkable properties that make them ideal for use in various industrial, technological, and scientific applications. From electronics to medicine, energy storage, and environmental remediation, Al2O3 nanoparticles are gaining widespread attention due to their high thermal stability, mechanical strength, chemical inertness, and biocompatibility. This article delves into the properties, synthesis techniques, and cutting-edge applications of Al2O3 nanoparticles.
What Are Al2O3 Nanoparticles?
Aluminum oxide (Al2O3) is a compound composed of aluminum and oxygen. It naturally occurs as bauxite ore and has been used in various forms, such as in abrasives, ceramics, and catalysts. When reduced to the nanoscale, Al2O3 nanoparticles exhibit unique properties compared to their bulk counterpart. These nanoparticles feature a high surface area, improved reactivity, and better mechanical properties, making them suitable for a wide range of applications.
In nanoparticle form, Al2O3 can have varying structures, including amorphous, gamma-alumina, alpha-alumina, and others, depending on the synthesis method. These structural variations contribute to their diverse applications.
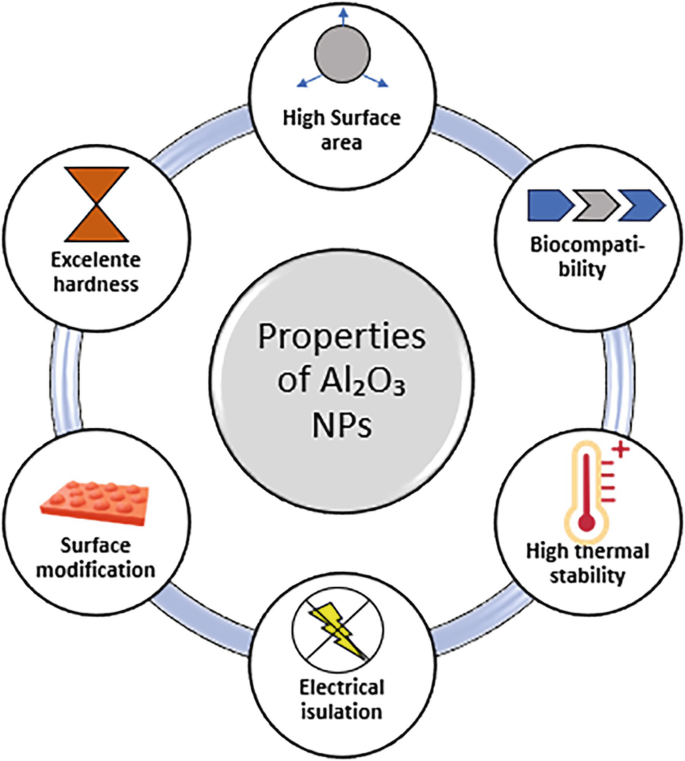
Properties of Al2O3 Nanoparticles
The unique properties of Al2O3 nanoparticles make them suitable for use in several industries. Some of the key properties include:
1. High Thermal Stability
Aluminum oxide nanoparticles can withstand extremely high temperatures. They have a melting point of over 2000°C, which makes them ideal for use in high-temperature environments such as thermal insulation and ceramic coatings.
- Example: Al2O3 nanoparticles are used in thermal management applications, including in thermal barrier coatings for turbines and engines.
2. Mechanical Strength
Al2O3 nanoparticles have remarkable mechanical properties that make them highly effective as reinforcements in composite materials. Their high hardness and abrasion resistance make them useful in applications that require durable, wear-resistant materials.
- Example: Used as abrasives in polishing materials and in wear-resistant coatings.
3. High Surface Area
Due to their nanoscale size, Al2O3 nanoparticles have a large surface area relative to their volume, making them ideal for applications where high surface interaction is necessary, such as in catalysis or adsorption processes.
- Example: Al2O3 nanoparticles are often used in catalysts for chemical reactions due to their high surface area and ability to provide active sites.
4. Chemical Inertness
Al2O3 is chemically stable and non-reactive, making it suitable for applications in aggressive chemical environments. This property is particularly valuable in catalysis, coatings, and pharmaceutical formulations.
- Example: Aluminum oxide nanoparticles are used as protective coatings for metal surfaces to prevent corrosion.
5. Biocompatibility
Al2O3 nanoparticles exhibit good biocompatibility and are widely used in medical applications such as drug delivery systems and implants. They do not provoke significant adverse biological reactions, making them suitable for direct interaction with living tissues.
- Example: Al2O3 nanoparticles are used in bone implants and medical prosthetics to enhance performance and reduce wear and tear.
6. UV Absorption
Aluminum oxide nanoparticles are known to have good UV absorption properties, making them ideal for use in sunscreens, coatings, and other UV-protective materials.
- Example: Al2O3 nanoparticles are used in cosmetic formulations and UV-blocking coatings for windows and automotive glass.
Synthesis Methods of Al2O3 Nanoparticles
Several synthesis methods are employed to produce Al2O3 nanoparticles, and the choice of method depends on the desired size, morphology, and application. Common synthesis techniques include:
1. Sol-Gel Method
The sol-gel process is widely used to synthesize Al2O3 nanoparticles due to its ability to produce high-purity materials. This method involves converting metal alkoxides into a sol (a colloidal suspension) and then forming a gel that is subsequently calcined to form Al2O3 nanoparticles.
- Advantages: Controlled particle size and morphology.
- Applications: Ceramics, coatings, and catalysis.
2. Hydrothermal Synthesis
In hydrothermal synthesis, Al2O3 nanoparticles are produced by reacting aluminum salts with water at high pressure and temperature. This method is often used to prepare crystalline Al2O3 with specific morphologies.
- Advantages: High crystallinity, controlled morphology.
- Applications: Catalysis, adsorption, and energy storage.
3. Chemical Vapor Deposition (CVD)
In CVD, aluminum precursors are vaporized and reacted with oxygen at high temperatures to form Al2O3 nanoparticles. This method allows for the production of high-quality, uniform nanoparticles with controlled properties.
- Advantages: High purity and uniformity.
- Applications: Coatings and semiconductor manufacturing.
4. Flame Synthesis
Flame synthesis involves the combustion of aluminum-based precursors to produce Al2O3 nanoparticles. The method is known for its simplicity and speed, allowing for the production of large quantities of nanoparticles.
- Advantages: Fast synthesis and scalability.
- Applications: Abrasive materials, coatings, and catalysts.
5. Ball Milling
Ball milling is a mechanical technique where aluminum powder is ground with oxidizing agents to produce Al2O3 nanoparticles. This method is simple and cost-effective, making it suitable for large-scale production.
- Advantages: Low-cost, scalable.
- Applications: Composites and abrasives.
Applications of Al2O3 Nanoparticles
Al2O3 nanoparticles have a wide range of applications across various industries due to their exceptional properties. Some notable applications include:
1. Catalysis and Adsorption
Al2O3 nanoparticles are widely used as catalysts and adsorbents due to their high surface area and chemical inertness. They are used in petroleum refining, chemical synthesis, and environmental applications like water purification.
- Example: Al2O3 nanoparticles serve as catalysts in hydrogenation reactions and dehydrogenation processes.
2. Thermal Barrier Coatings
Due to their high thermal stability, Al2O3 nanoparticles are used in thermal barrier coatings for engines, turbines, and high-temperature equipment. These coatings protect materials from heat damage and improve their efficiency and lifespan.
- Example: Al2O3 nanoparticle coatings are used in aerospace and automotive industries to withstand high operating temperatures.
3. Biomedical Applications
Al2O3 nanoparticles are biocompatible, making them suitable for medical applications such as bone implants, prosthetics, and drug delivery systems. They enhance the mechanical properties of implants and provide controlled release of drugs.
- Example: Al2O3 nanoparticles are used in hip replacement and dental implants for their strength and biocompatibility.
4. Electronics and Semiconductors
In electronics, Al2O3 nanoparticles are used as insulating materials, dielectrics, and in the production of semiconductors. Their insulating properties make them ideal for use in capacitors and transistors.
- Example: Al2O3 nanoparticles are used in electronic devices as dielectric materials and insulators.
5. UV Protection
Al2O3 nanoparticles exhibit excellent UV absorption properties, making them ideal for use in sunscreens, cosmetics, and UV-blocking coatings.
- Example: Al2O3 nanoparticles are used in UV-protective coatings for windows and automotive glass to block harmful UV rays.
6. Wear-resistant Coatings
The high hardness of Al2O3 nanoparticles makes them perfect for use in wear-resistant coatings for machinery, tools, and cutting instruments.
- Example: Al2O3 nanoparticle-based coatings are applied to cutting tools and machine parts to increase their durability and performance.
Conclusion
Al2O3 nanoparticles are an incredibly versatile material with a broad range of applications across multiple industries. Their thermal stability, mechanical strength, chemical inertness, and biocompatibility make them ideal for use in everything from energy storage and catalysis to biomedical devices and coatings. As the demand for high-performance materials grows, Al2O3 nanoparticles will continue to play an essential role in advancing technologies and enabling more sustainable, efficient solutions in various fields.
