Properties and Applications of Aluminum Oxide Nanoparticles
Introduction
Aluminum oxide (Al₂O₃) nanoparticles, commonly known as alumina nanoparticles, are materials that have garnered significant interest in both academic and industrial research due to their unique physical, chemical, and mechanical properties. These nanoparticles exhibit remarkable versatility, making them ideal for various applications, including electronics, catalysis, biomedical, and environmental fields.
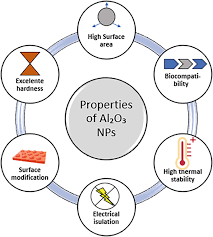
Properties of Aluminum Oxide Nanoparticles
- Size and Surface Area
- Nanometer Scale: The primary defining characteristic of aluminum oxide nanoparticles is their size, typically ranging from 1 to 100 nm. At this scale, the particles exhibit quantum effects, significantly enhancing their chemical reactivity and surface interactions compared to bulk Al₂O₃.
- High Surface Area: Due to their small size and high surface-to-volume ratio, aluminum oxide nanoparticles have an elevated surface area that contributes to their increased reactivity, adsorption, and catalytic properties.
- Chemical Stability
- Corrosion Resistance: Al₂O₃ nanoparticles are highly stable and resistant to corrosion, which makes them durable in harsh environmental conditions. This feature is one of the reasons why they are extensively used in coatings and electronics.
- High Temperature Stability: Aluminum oxide nanoparticles can withstand high temperatures, often up to 1000°C or more, without decomposing, making them valuable in high-temperature applications.
- Surface Reactivity: These nanoparticles can have hydroxyl groups on their surfaces, which may participate in a range of chemical reactions, such as adsorption of water, ions, or organic molecules.
- Mechanical Properties
- Hardness: Aluminum oxide is a hard, abrasive material, with a Mohs hardness of 9, which is second only to diamonds. This property makes Al₂O₃ nanoparticles useful in polishing and coating applications.
- High Fracture Toughness: Al₂O₃ nanoparticles exhibit improved mechanical strength compared to bulk aluminum oxide, making them suitable for reinforcing composite materials.
- Optical Properties
- Transparency: Alumina is transparent in certain forms, particularly in the ultraviolet (UV) spectrum. This property is valuable for optoelectronic and photonic applications.
- High Refractive Index: The nanoparticles exhibit a high refractive index, which is beneficial in optical coatings and other light-manipulating technologies.
- Electrical Insulation
- Aluminum oxide is an excellent electrical insulator, which makes its nanoparticles useful in electronic devices, sensors, and insulating materials.
- Biocompatibility
- Aluminum oxide nanoparticles are generally non-toxic and biocompatible, making them suitable for biomedical applications, including drug delivery, wound healing, and imaging.
Synthesis Methods of Aluminum Oxide Nanoparticles
Several techniques can be used to synthesize aluminum oxide nanoparticles, each offering control over the size, morphology, and properties of the particles:
- Sol-Gel Process: This involves the formation of a gel from an aluminum precursor, followed by drying and heat treatment to obtain Al₂O₃ nanoparticles. It is highly controlled and allows for the production of uniform particles.
- Hydrothermal Synthesis: A method that uses high pressure and temperature to synthesize nanoparticles from a solution containing aluminum salts. This technique typically yields nanoparticles with high crystallinity.
- Chemical Vapor Deposition (CVD): This method involves the deposition of aluminum oxide from gaseous precursors onto a substrate. CVD is suitable for producing thin films and coating applications.
- Laser Ablation: A technique that uses laser pulses to ablate a target material (e.g., bulk aluminum) in a liquid medium to produce nanoparticles. This method is useful for obtaining nanoparticles with high purity and control over size.
- Ball Milling: A mechanical process where bulk Al₂O₃ is ground into nanoparticles. While simple, this method may lead to less controlled particle sizes compared to chemical methods.
Applications of Aluminum Oxide Nanoparticles
- Catalysis and Catalysis Support
- Catalysts: Aluminum oxide nanoparticles are used as catalysts or catalyst supports in various chemical reactions, such as in petroleum refining and the production of chemicals like methanol. Their high surface area and thermal stability enhance the efficiency of catalytic processes.
- Environmental Catalysis: Al₂O₃ nanoparticles play a role in environmental cleanup, such as in the degradation of pollutants or in catalytic converters for automobile emissions.
- Biomedical Applications
- Drug Delivery: Due to their biocompatibility and surface reactivity, aluminum oxide nanoparticles can be functionalized to deliver drugs to targeted sites within the body. They are also used as carriers for proteins, genes, and other therapeutic agents.
- Wound Healing: Al₂O₃ nanoparticles have been explored for their ability to promote wound healing. Their antimicrobial properties help reduce infections while promoting faster tissue regeneration.
- Imaging and Diagnostic Tools: Due to their optical properties, these nanoparticles can be used in medical imaging (e.g., MRI contrast agents) and diagnostics.
- Electronics and Photonics
- Insulating Materials: The high electrical insulation properties of aluminum oxide nanoparticles make them useful in the fabrication of semiconductors, insulators, and other electronic components.
- Optical Coatings: The transparency and high refractive index of Al₂O₃ nanoparticles are exploited in optical coatings for lenses, mirrors, and other optical devices.
- Energy Storage and Batteries
- Supercapacitors: Al₂O₃ nanoparticles are used as components in supercapacitors and batteries, improving charge storage and stability.
- Fuel Cells: Aluminum oxide nanoparticles also serve as catalysts in fuel cells, enhancing efficiency and longevity.
- Environmental Applications
- Water Purification: Due to their high surface area, aluminum oxide nanoparticles are used for the removal of heavy metals, organic pollutants, and other contaminants from water, making them useful in water treatment and purification.
- Air Filtration: Al₂O₃ nanoparticles are incorporated into air filtration systems to remove particulate matter and other pollutants from the air.
- Cosmetic and Personal Care Products
- Sunscreens and Antioxidants: Due to their transparency and UV-absorbing properties, aluminum oxide nanoparticles are used in sunscreens, providing protection from harmful UV radiation without the opaque appearance of traditional zinc oxide or titanium dioxide-based products.
- Polishing Agents: Their hardness and abrasiveness make Al₂O₃ nanoparticles ideal for use in facial scrubs, toothpaste, and polishing products.
- Composite Materials
- Reinforcement in Polymers and Metals: Aluminum oxide nanoparticles are used to reinforce composite materials, such as polymer matrices and metal alloys. Their high strength and durability improve the mechanical properties and thermal stability of these materials.
- Ceramics and Refractories: Al₂O₃ nanoparticles are also used in the production of advanced ceramics and refractories, which are crucial in industries requiring high-temperature resistance.
Challenges and Future Directions
Despite the vast potential of aluminum oxide nanoparticles, there are several challenges associated with their use:
- Toxicity and Environmental Concerns: The impact of Al₂O₃ nanoparticles on human health and the environment remains a topic of ongoing research. While they are generally considered biocompatible, their long-term effects, especially when they accumulate in the body, need further investigation.
- Scalability and Cost: Some of the synthesis methods for aluminum oxide nanoparticles are complex and expensive, limiting their widespread industrial application. Advances in scalable production techniques are essential to reduce costs and increase availability.
- Functionalization and Surface Modification: To enhance the performance of aluminum oxide nanoparticles in specific applications, surface modification techniques must be developed to tailor their properties (e.g., hydrophobicity, drug delivery, and catalysis efficiency).
Conclusion
Aluminum oxide nanoparticles possess a combination of unique properties, such as high surface area, chemical stability, mechanical strength, and biocompatibility, which make them suitable for a wide range of applications. From catalysis to biomedical uses, their potential continues to expand with ongoing research. However, challenges related to scalability, toxicity, and cost remain, and addressing these issues will be critical to realizing the full potential of aluminum oxide nanoparticles in various industrial and scientific domains.