How to Make a Lithium-Ion Coin Cell Battery
Lithium-ion coin cell batteries are compact, efficient, and widely used in various applications such as watches, key fobs, hearing aids, and even small medical devices. These batteries are highly favored for their high energy density and long shelf life. This article provides a step-by-step guide on how to make a lithium-ion coin cell battery from scratch, including the essential materials, tools, and procedures involved.
Materials Needed
Before starting, it is important to gather the necessary materials for making a lithium-ion coin cell battery. The components typically include:
1. Electrodes
- Cathode material: Lithium cobalt oxide (LiCoO₂) or LiFePO₄ are commonly used in coin cells.
- Anode material: Graphite is the most commonly used anode material in lithium-ion batteries.
2. Electrolyte
- Lithium salt solution (such as LiPF₆ in a solvent like ethylene carbonate (EC) and dimethyl carbonate (DMC)) is typically used as the electrolyte to enable ion movement.
3. Separator
- A polyethylene (PE) or polypropylene (PP) separator is used to prevent internal short-circuiting by physically separating the anode and cathode while still allowing ion flow.
4. Coin Cell Case
- A stainless steel coin cell case is used to house the electrodes, separator, and electrolyte.
5. Other Materials
- Current collectors: Aluminum for the cathode and copper for the anode.
- Gaskets: Used to seal the cell and ensure no leakage of electrolyte.
- Sealants: Typically a rubber or polymer gasket to create an airtight seal.
Tools Needed
- Coin cell crimping machine or hand tools for sealing.
- Electrode punching machine or scissors for cutting electrodes to size.
- Vacuum chamber or a glove box to handle the assembly in a dry, controlled atmosphere.
- Gloves to prevent contamination of materials.
- Micrometer to measure electrode thickness.
- Cell tester to monitor the performance of the assembled cell.
Step-by-Step Guide to Making a Lithium-Ion Coin Cell Battery
Step 1: Preparation of the Electrodes
To make a lithium-ion coin cell, you first need to prepare the cathode and anode electrodes:
Cathode Preparation:
- Mix the active material (e.g., LiCoO₂), binder (such as polyvinylidene fluoride, or PVDF), and a conductive agent (such as carbon black) into a slurry.
- Spread the slurry onto a metal foil substrate, usually made of aluminum, which will serve as the current collector.
- Dry the coated foil at a temperature of about 120°C for 12-24 hours to remove any residual solvent.
- Cut the dried electrode into small, circular discs that will fit into the coin cell case.
Anode Preparation:
- Prepare the anode material, which is typically graphite mixed with a binder (like CMC or carboxymethyl cellulose).
- Spread the slurry onto a copper foil to serve as the anode current collector.
- Dry and cut the anode in the same way as the cathode.
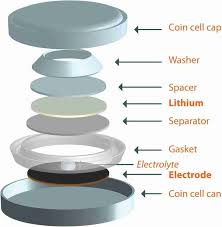
Step 2: Assemble the Coin Cell
The next step involves the actual assembly of the coin cell.
- Place the cathode in the bottom of the coin cell case, ensuring it is aligned with the current collector.
- Place the separator (typically a polymer membrane) on top of the cathode. The separator should cover the entire surface of the cathode and be thin enough to allow lithium-ion movement but strong enough to prevent physical contact between the electrodes.
- Place the anode on top of the separator. Ensure it fits snugly inside the coin cell and does not shift out of position.
- Add electrolyte:
- Inject the liquid electrolyte (LiPF₆ in a solvent mixture like EC and DMC) into the cell. Ensure the separator is completely saturated with the electrolyte.
- The amount of electrolyte is crucial: too much and the cell could leak, too little and the battery won’t perform as expected.
- Seal the coin cell:
- Use a crimping tool to seal the coin cell. The crimping process secures the anode, cathode, separator, and electrolyte in place.
- Ensure a tight seal to avoid electrolyte leakage and ensure proper operation.
Step 3: Testing and Formation Cycle
Once the coin cell is sealed, it’s important to test and condition it to ensure it functions properly.
- Initial charging:
- Use a battery charger to perform the initial charge cycle, often referred to as formation cycling. This step ensures the battery reaches its full charge capacity and helps condition the electrodes.
- Test the voltage:
- A fully charged lithium-ion coin cell should have a voltage of about 3.7V. Test the cell with a voltmeter to confirm the voltage.
- Capacity testing:
- Discharge the cell using a battery tester or constant current load tester to check the battery’s capacity and performance.
- Ideally, the coin cell should show a consistent voltage drop when discharged and should charge back up to full capacity after each cycle.
Step 4: Final Testing and Quality Control
After the coin cell is fully assembled and conditioned, you should carry out final tests to ensure that the battery meets the required specifications:
- Leakage test: Ensure there are no electrolyte leaks from the coin cell casing.
- Internal resistance test: Use an electrical impedance tester to check for any abnormal internal resistance.
- Cycle life test: To test the longevity of your coin cell, subject it to multiple charge/discharge cycles to observe how well it holds up over time.
Safety Considerations
Making a lithium-ion coin cell battery involves working with potentially hazardous materials and equipment. Here are some important safety precautions to keep in mind:
- Work in a dry environment: Lithium-ion batteries are sensitive to moisture, so it’s important to assemble the cells in a dry room or glove box to prevent moisture contamination, which can cause short-circuiting or reduced performance.
- Handle with care: Lithium-ion batteries contain flammable materials. Always handle the cell with care, avoiding punctures, physical stress, or short-circuiting.
- Use appropriate PPE (Personal Protective Equipment): Wear gloves, safety glasses, and a lab coat to prevent direct contact with the chemicals and materials used in the battery.
- Dispose of waste materials safely: Follow local regulations for the disposal of chemicals, solvents, and used battery cells.
Conclusion
Making a lithium-ion coin cell battery involves a detailed process of preparing the electrodes, assembling the components, and conditioning the cell to ensure high performance. While the process can be complex, it provides valuable insight into how lithium-ion batteries work and the materials that make them so efficient. Whether you’re a DIY enthusiast or a researcher looking to understand battery technology better, this guide gives you a comprehensive overview of how to make your own coin cell battery.
As technology continues to advance, further improvements in lithium-ion battery manufacturing will lead to even more efficient, durable, and environmentally friendly batteries in the future.
