Iron Nanoparticles: Properties and Applications
Iron nanoparticles (FeNPs) have garnered significant attention in recent years due to their unique properties and diverse range of applications in various industries. These nanoparticles, due to their small size, exhibit enhanced reactivity, surface area, and magnetic properties compared to their bulk counterparts, making them ideal for a variety of uses, from medical treatments to environmental remediation and energy storage. In this article, we will explore the properties of iron nanoparticles and their applications in different sectors.
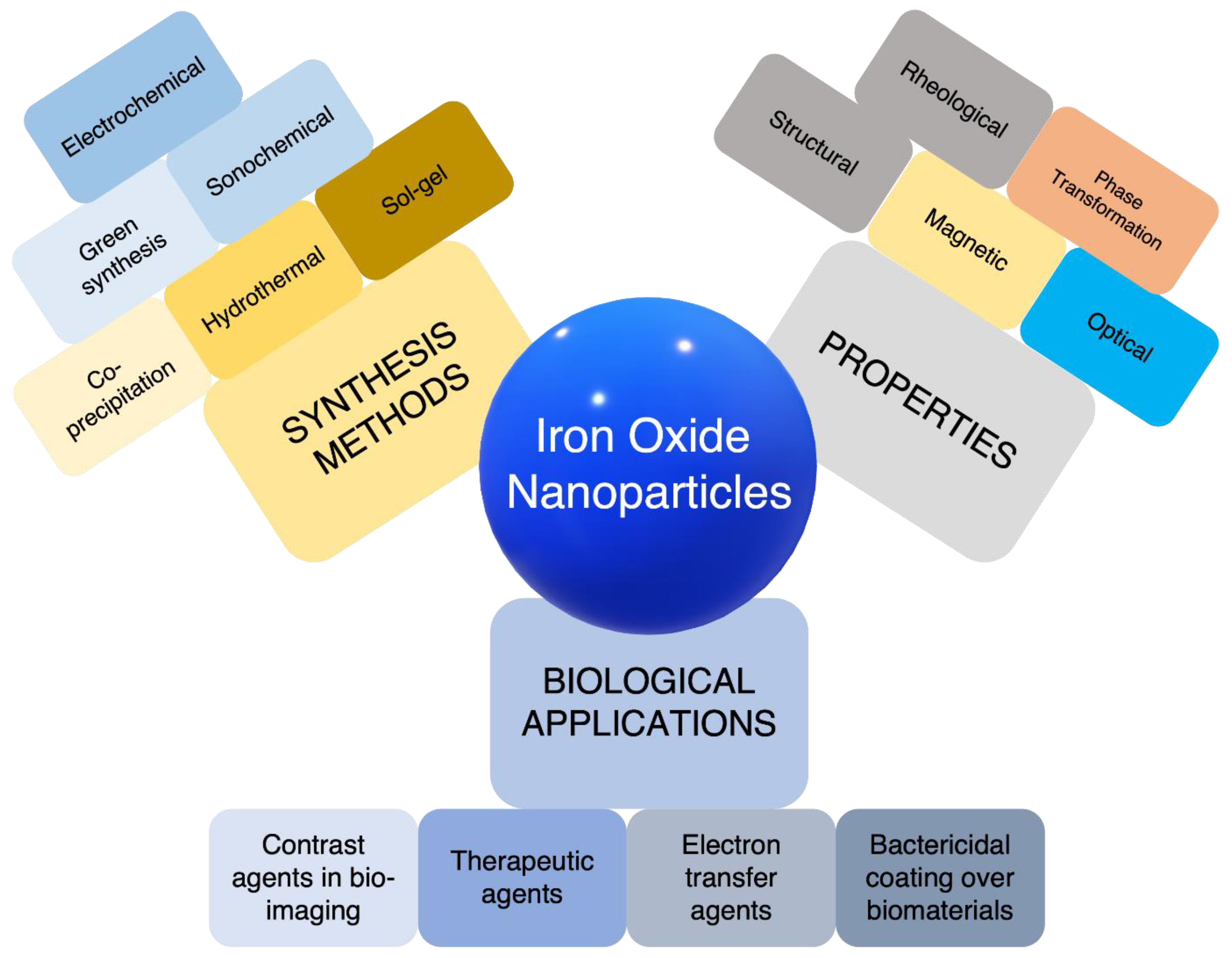
1. What are Iron Nanoparticles?
Iron nanoparticles are tiny particles of iron that typically range in size from 1 to 100 nanometers (nm). At the nanoscale, iron exhibits properties that are distinct from its bulk form, including increased surface area, enhanced chemical reactivity, and unique physical properties such as superparamagnetism. These characteristics make iron nanoparticles highly valuable in numerous applications, especially where high reactivity, magnetism, or a combination of both is desired.
There are different forms of iron nanoparticles, including:
- Zero-valent iron nanoparticles (Fe0): These are unoxidized iron particles with the potential for environmental applications such as water treatment and remediation.
- Iron oxide nanoparticles (Fe3O4, Fe2O3): These are iron particles that have been oxidized to form iron oxide and are commonly used for biomedical and magnetic applications.
2. Properties of Iron Nanoparticles
a. Magnetic Properties
One of the most prominent characteristics of iron nanoparticles is their magnetic behavior. Iron nanoparticles, particularly Fe3O4 (magnetite), exhibit superparamagnetism, a phenomenon where the nanoparticles are magnetized only in the presence of an external magnetic field and lose their magnetization once the field is removed. This property is useful in various applications, including:
- Magnetic separation: In biomedical and environmental applications, iron nanoparticles can be used for magnetic separation of particles or contaminants.
- Magnetic resonance imaging (MRI): Due to their magnetic properties, iron oxide nanoparticles can be used as contrast agents in MRI, allowing for improved imaging of tissues.
b. High Surface Area and Reactivity
Due to their small size and large surface area to volume ratio, iron nanoparticles exhibit enhanced chemical reactivity compared to bulk iron. This makes them highly effective in catalysis, environmental remediation, and energy storage applications. The increased surface area allows for greater interaction with surrounding materials, improving reaction rates and efficiency.
c. Biocompatibility and Non-toxicity
Iron nanoparticles, particularly those that are oxidized to form iron oxide (Fe3O4), are considered biocompatible and generally non-toxic, which makes them ideal for use in biomedical applications. They can be engineered for various purposes, such as drug delivery, biosensing, and imaging.
d. Tunable Properties
The properties of iron nanoparticles, such as size, surface charge, and composition, can be easily tuned by adjusting synthesis methods. This ability to modify the nanoparticles allows for fine control over their behavior and functionality in various applications, making them highly versatile.
3. Applications of Iron Nanoparticles
a. Environmental Remediation
One of the most significant applications of iron nanoparticles is in environmental remediation, particularly in the treatment of water and soil contaminated with hazardous substances. Zero-valent iron nanoparticles (Fe0) are particularly useful for breaking down toxic compounds, such as:
- Heavy metals: Iron nanoparticles can effectively remove metals like arsenic, chromium, and lead from contaminated water through reduction reactions.
- Organic pollutants: Iron nanoparticles are used to break down organic contaminants like pesticides, chlorinated solvents, and oil spills through chemical reactions.
Iron nanoparticles can be used in in situ applications, where they are directly injected into contaminated sites to neutralize pollutants without needing to remove the contaminated material.
b. Biomedical Applications
Iron nanoparticles, particularly iron oxide nanoparticles, have found extensive applications in the biomedical field due to their biocompatibility and magnetic properties. Some key uses include:
- Drug Delivery: Iron nanoparticles can be loaded with drugs and directed to specific sites in the body using an external magnetic field. This magnetic targeting improves the efficiency of the drug delivery system and reduces side effects, especially in cancer therapies.
- Magnetic Resonance Imaging (MRI): Iron oxide nanoparticles serve as contrast agents in MRI, enhancing the resolution and sensitivity of the imaging process. Their ability to be magnetized makes them highly effective in detecting tissue abnormalities or tumors.
- Hyperthermia Treatment: Iron nanoparticles are used in magnetic hyperthermia, a cancer treatment method where nanoparticles are exposed to an alternating magnetic field, causing them to heat up and destroy cancer cells through localized heating.
- Biosensors: Iron nanoparticles are also used in the development of biosensors for detecting specific biomarkers or pathogens in medical diagnostics.
c. Catalysis
Iron nanoparticles are increasingly used as catalysts in various chemical reactions, owing to their high surface area and reactivity. Some applications include:
- Fischer-Tropsch synthesis: Iron nanoparticles are used as catalysts in the conversion of carbon monoxide and hydrogen into liquid hydrocarbons, which can be used as a synthetic fuel.
- Catalysis for CO2 reduction: Iron nanoparticles are being explored for their potential to catalyze the reduction of carbon dioxide (CO2) into valuable chemicals, such as methane or ethylene, which could have significant implications for carbon capture and storage technologies.
d. Energy Storage and Conversion
Iron nanoparticles have a growing presence in energy storage and conversion systems due to their high reactivity and potential for battery applications. Some notable uses include:
- Lithium-ion batteries: Iron-based nanoparticles, such as Fe3O4, are being explored as anodes in lithium-ion batteries, offering advantages in terms of cost, availability, and performance.
- Supercapacitors: Iron oxide nanoparticles are used in supercapacitors as electrodes, where their large surface area enhances charge storage and boosts the efficiency of energy storage devices.
- Hydrogen storage: Iron nanoparticles are also being researched for their ability to store hydrogen, an essential component of hydrogen fuel cells.
e. Magnetic Separation and Sensing
Due to their superparamagnetic properties, iron nanoparticles are widely used in magnetic separation techniques to isolate specific substances, such as proteins, nucleic acids, or bacteria. In these applications, the particles can be functionalized with specific ligands or antibodies to target certain molecules. They are also used in magnetic sensors to detect changes in magnetic fields, enabling their use in biosensing and environmental monitoring.
f. Agriculture
Iron nanoparticles can also play a role in agriculture. Their high surface area and ability to adsorb nutrients or pesticides can be used to enhance fertilizer delivery, increase plant growth through nutrient management, or as part of nano-pesticide formulations that target pests more effectively while minimizing environmental impact.
4. Challenges and Future Directions
Despite the promising applications, there are still some challenges associated with iron nanoparticles:
- Toxicity and Environmental Impact: While iron oxide nanoparticles are generally considered biocompatible, the potential long-term effects of iron nanoparticles, especially those that are not fully oxidized, need further investigation. The potential for nanoparticle accumulation in organisms and ecosystems is an area of concern.
- Scalability: Although iron nanoparticles have shown great promise in laboratory settings, scaling up production methods for industrial use remains a challenge. Researchers are working on optimizing synthesis processes to ensure the cost-effective production of iron nanoparticles at large scales.
5. Conclusion
Iron nanoparticles are an exciting class of materials with a wide range of applications across diverse fields, including environmental remediation, biomedicine, energy storage, and catalysis. Their unique properties, such as high surface area, enhanced reactivity, and magnetic behavior, make them invaluable for addressing challenges in technology and sustainability. As research continues and new synthesis methods are developed, the use of iron nanoparticles is expected to expand, providing innovative solutions for a variety of industries and applications.