Role of Nanomaterials in Catalysis
Nanomaterials have emerged as a significant class of materials with unique properties that make them highly effective in catalysis. Their exceptional characteristics—such as high surface area, small size, tunable composition, and the ability to modify their electronic and structural properties—make them ideal candidates for use as catalysts in a wide range of chemical reactions. This article explores the role of nanomaterials in catalysis, their types, mechanisms, and potential applications.
1. Unique Properties of Nanomaterials
Nanomaterials typically range in size from 1 to 100 nanometers, and their properties can differ significantly from those of bulk materials. These unique properties include:
- High Surface Area: Nanomaterials have a much higher surface-to-volume ratio compared to bulk materials, which provides more active sites for catalytic reactions.
- Quantum Effects: At the nanoscale, quantum effects begin to play a role in the behavior of the material, influencing electronic, optical, and magnetic properties. This can enhance catalytic performance.
- High Reactivity: The increased number of atoms on the surface of nanomaterials leads to higher reactivity, which is essential in catalytic processes.
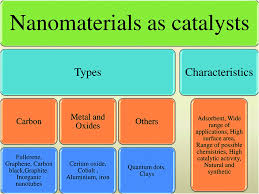
2. Types of Nanomaterials Used in Catalysis
Several types of nanomaterials are employed in catalysis, each offering distinct advantages depending on the reaction conditions and the nature of the reaction:
- Metal Nanoparticles: These are one of the most widely studied types of nanomaterials in catalysis. Metals like platinum, palladium, gold, and silver exhibit high catalytic activity for a variety of reactions such as hydrogenation, oxidation, and CO oxidation.
- Metal Oxides: Nanostructured metal oxides like TiO₂, CeO₂, and ZnO are used in catalytic applications due to their stability, versatility, and the ability to support other catalytic species.
- Carbon-Based Nanomaterials: Carbon nanotubes (CNTs) and graphene are known for their exceptional mechanical strength, electrical conductivity, and large surface area. They are often used as supports for other catalysts or as direct catalysts in reactions like hydrogenation and dehydrogenation.
- Layered Double Hydroxides (LDHs): These materials have gained attention for their ability to host various metal ions, which can participate in catalytic reactions.
- Metal-Organic Frameworks (MOFs): MOFs are highly porous materials with a tunable structure, which makes them ideal candidates for catalytic applications, particularly in gas-phase reactions and storage applications.
3. Mechanisms of Nanomaterials in Catalysis
Nanomaterials enhance catalysis through several mechanisms:
- Increased Active Site Density: The increased surface area of nanomaterials provides more active sites for the catalytic reaction to occur. This can lead to a higher reaction rate and better efficiency.
- Size-Dependent Properties: The small size of nanomaterials allows for the modification of their electronic properties. For example, metal nanoparticles exhibit size-dependent electronic properties, which can be fine-tuned for specific catalytic reactions.
- Surface Defects: Nanomaterials often have defects and irregularities on their surfaces, such as vacancies or steps, which can act as additional active sites for catalysis.
- Support Effect: Nanomaterials can act as supports for other catalysts, stabilizing and dispersing them to prevent sintering and deactivation. For instance, nanoparticles of precious metals can be supported on carbon or metal oxides to improve stability and performance.
4. Applications of Nanomaterials in Catalysis
Nanomaterials are used in a wide range of catalytic applications, including:
- Energy Conversion and Storage: Nanocatalysts play a vital role in fuel cells, hydrogen production, and storage, as well as in batteries and supercapacitors. For example, platinum-based nanoparticles are commonly used as electrocatalysts in fuel cells.
- Environmental Catalysis: Nanomaterials are employed in the removal of pollutants from air and water. They are used in processes like catalytic oxidation to degrade toxic chemicals, or in selective catalytic reduction (SCR) to reduce nitrogen oxide (NOx) emissions from vehicle exhausts.
- Green Chemistry: Nanocatalysts are often utilized in environmentally friendly processes, such as green hydrogen production through water splitting or in the production of biofuels from renewable resources.
- Fine Chemicals and Pharmaceuticals: Nanocatalysts are crucial in fine chemical production, such as the synthesis of pharmaceuticals and agrochemicals. Their ability to provide high specificity and selectivity in reactions makes them valuable in this area.
5. Challenges and Future Prospects
Despite the numerous advantages of nanomaterials in catalysis, several challenges remain:
- Synthesis and Scalability: The production of nanomaterials with consistent quality and reproducibility can be difficult. Scaling up the synthesis of these materials while maintaining their catalytic properties is a key challenge.
- Stability and Durability: Nanomaterials, especially metal nanoparticles, can suffer from issues like sintering, leaching, or deactivation during catalytic processes. Developing strategies to stabilize these materials is crucial.
- Cost: While nanomaterials can offer improved performance, their cost, especially for precious metal nanoparticles, can be prohibitive for large-scale industrial applications.
- Environmental Impact: The potential environmental impact of nanomaterials, including toxicity and the disposal of waste products, needs to be carefully considered as their use in industrial processes expands.
6. Conclusion
Nanomaterials play a pivotal role in advancing catalytic technologies due to their unique properties that enhance reactivity, selectivity, and efficiency. With continued research and innovation, nanomaterials are poised to revolutionize catalysis in diverse fields, including energy conversion, environmental protection, and industrial chemical production. Overcoming the challenges related to scalability, stability, and cost will be crucial for their broader adoption in industrial catalysis. As our understanding of these materials deepens, their potential to provide sustainable and efficient solutions to global challenges will only increase.
